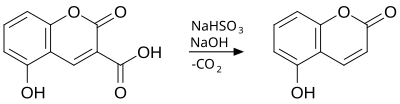
The Adams decarboxylation is a chemical reaction that involved the decarboxylation of coumarins which have carboxylic acid group in the third position. The decarboxylation is achieved by aqueous solution of sodium bisulfite, heat and a concentrated solution of sodium hydroxide.
References
- Adams, R.; Mathieu, J. (1948). "A New Synthesis of Atranol (2,6-Dihydroxy-4-methylbenzaldehyde) and the Corresponding Cinnamic Acid". J. Am. Chem. Soc. 70 (6): 2120. doi:10.1021/ja01186a037.
- Adams, R.; Bockstaheler, T.E. (1952). "Preparation and Reactions of o-Hydroxycinnamic Acids and Esters". J. Am. Chem. Soc. 74 (21): 5346. doi:10.1021/ja01141a038.
- Cramer, F.; Windel, H. (1956). "Über Einschlußverbindungen, X. Mitteil.: Die blauen Jodverbindungen der Cumarine und anderer verwandter Verbindungen". Chem. Ber. 89 (2): 354. doi:10.1002/cber.19560890227.