| Nierenstein reaction | |
|---|---|
| Named after | Maximilian Nierenstein |
| Reaction type | Carbon-carbon bond forming reaction |
The Nierenstein reaction is an organic reaction describing the conversion of an acid chloride into a haloketone with diazomethane. It is an insertion reaction in that the methylene group from the diazomethane is inserted into the carbon-chlorine bond of the acid chloride.

Reaction mechanism
The reaction proceeds through a diazonium salt intermediate formed by displacement of the chloride with diazomethyl anion.
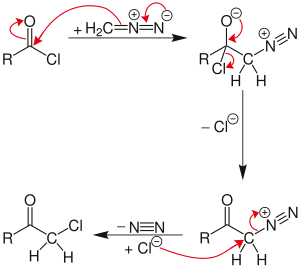
If excess diazomethane is present during the reaction, it can act as a base, abstracting a hydrogen from the diazonium-salt intermediate. The result is a neutral diazoketone, which does not react with the chloride. Instead, the byproduct, diazonium-methyl from the other diazomethane molecule, can be attacked by the chloride to produce chloromethane. The unreactive diazoketone can be re-activated and reacted by treatment with hydrogen chloride to give the normal Nierenstein product.

In some cases, even limiting the amount of diazomethane gives a reaction process that stalls via the neutral diazoketone pathway, requiring the addition of HCl gas to rescue it.
Scope
One original 1924 Nierenstein reaction:

and a reaction starting from benzoyl bromide going haywire with formation of the dioxane dimer:

See also
- Maximilian Nierenstein
- Curtius rearrangement
- Wolff rearrangement
- Arndt–Eistert reaction: where acid chlorides react with diazomethane to give chain extended carboxylic acids via a rearrangement
References
- Clibbens, D.; Nierenstein, M. (1915). "The action of diazomethane on some aromatic acyl chlorides". J. Chem. Soc. 107: 1491. doi:10.1039/CT9150701491.
- Bachman, W. E.; Struve, W. S. (1942). "The Arndt-Eistert Reaction". Org. React. 1: 38. (Review)
- McPhee, W. D; Klingsberg, E. Organic Syntheses, Coll. Vol. 3, p.119 (1955); Vol. 26, p.13 (1946). (Article)
- M. Nierenstein; D. G. Wang & J. C. Warr (1924). "The Action of Diazomethane on some Aromatic Acyl Chlorides II. Synthesis of Fisetol". J. Am. Chem. Soc. 46 (11): 2551–2555. doi:10.1021/ja01676a028.
- H. H. Lewis; M. Nierenstein & Enid M. Rich (1925). "The Action of Diazomethane on some Aromatic Acyl Chlorides III. The Mechanism of the Reaction". J. Am. Chem. Soc. 47 (6): 1728–1732. doi:10.1021/ja01683a036.