| ||||||||||||||||||||||||||||||||||
Americium (95Am) is an artificial element, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no known stable isotopes. The first isotope to be synthesized was Am in 1944. The artificial element decays by ejecting alpha particles. Americium has an atomic number of 95 (the number of protons in the nucleus of the americium atom). Despite
Am being an order of magnitude longer lived than
Am, the former is harder to obtain than the latter as more of it is present in spent nuclear fuel.
Eighteen radioisotopes of americium, ranging from Am to Am with the exception of Am, have been characterized; another isotope, Am, has also been reported but is unconfirmed. The most stable isotopes are Am with a half-life of 7,370 years and Am with a half-life of 432.2 years. All of the remaining radioactive isotopes have half-lives that are less than 51 hours, and the majority of these have half-lives that are less than 100 minutes. This element also has 8 meta states, with the most stable being Am (t1/2 = 141 years). This isomer is unusual in that its half-life is far longer than that of the ground state of the same isotope.
List of isotopes
| Nuclide |
Z | N | Isotopic mass (Da) |
Half-life |
Decay mode |
Daughter isotope |
Spin and parity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excitation energy | |||||||||||||||||||
| Am | 95 | 128 | 223.04584(32)# | 10(9) ms | α | Np | 9/2–# | ||||||||||||
| Am | 95 | 134 | 229.04528(11) | 1.8(15) s | α | Np | 5/2–# | ||||||||||||
| Am | 95 | 135 | 230.04603(15)# | 40(9) s | β (<70%) | Pu | 1–# | ||||||||||||
| βSF (>30%) | (various) | ||||||||||||||||||
| Am | 95 | 137 | 232.04661(32)# | 1.31(4) min | β (97%) | Pu | 1–# | ||||||||||||
| α (3%) | Np | ||||||||||||||||||
| βSF (0.069%) | (various) | ||||||||||||||||||
| Am | 95 | 138 | 233.04647(12)# | 3.2(8) min | β (95.5%) | Pu | 5/2–# | ||||||||||||
| α (4.5%) | Np | ||||||||||||||||||
| Am | 95 | 139 | 234.04773(17)# | 2.32(8) min | β (99.95%) | Pu | 0–# | ||||||||||||
| α (0.039%) | Np | ||||||||||||||||||
| β, SF (0.0066%) | (various) | ||||||||||||||||||
| Am | 95 | 140 | 235.047906(57) | 10.3(6) min | β (99.60%) | Pu | 5/2−# | ||||||||||||
| α (0.40%) | Np | ||||||||||||||||||
| Am | 95 | 141 | 236.04943(13)# | 3.6(1) min | β | Pu | 5− | ||||||||||||
| α (4×10%) | Np | ||||||||||||||||||
| Am | 50(50)# keV | 2.9(2) min | β | Pu | (1−) | ||||||||||||||
| α ? | Np | ||||||||||||||||||
| Am | 95 | 142 | 237.049995(64)# | 73.6(8) min | β (99.975%) | Pu | 5/2− | ||||||||||||
| α (.025%) | Np | ||||||||||||||||||
| Am | 95 | 143 | 238.051983(63) | 98(3) min | β | Pu | 1+ | ||||||||||||
| α (1.0×10%) | Np | ||||||||||||||||||
| Am | 2500(200)# keV | 35(18) μs | SF | (various) | |||||||||||||||
| IT ? | Am | ||||||||||||||||||
| Am | 95 | 144 | 239.0530227(21) | 11.9(1) h | EC (99.99%) | Pu | 5/2− | ||||||||||||
| α (0.01%) | Np | ||||||||||||||||||
| Am | 2500(200) keV | 163(12) ns | SF | (various) | (7/2+) | ||||||||||||||
| IT ? | Am | ||||||||||||||||||
| Am | 95 | 145 | 240.055298(15) | 50.8(3) h | β | Pu | (3−) | ||||||||||||
| α (1.9×10%) | Np | ||||||||||||||||||
| Am | 3000(200) keV | 940(40) μs | SF | (various) | |||||||||||||||
| IT ? | Am | ||||||||||||||||||
| Am | 95 | 146 | 241.0568273(12) | 432.6(6) y | α | Np | 5/2− | ||||||||||||
| SF (3.6×10%) | (various) | ||||||||||||||||||
| Am | 2200(200) keV | 1.2(3) μs | SF | (various) | |||||||||||||||
| Am | 95 | 147 | 242.0595474(12) | 16.02(2) h | β (82.7%) | Cm | 1− | ||||||||||||
| EC (17.3%) | Pu | ||||||||||||||||||
| Am | 48.60(5) keV | 141(2) y | IT (99.54%) | Am | 5− | ||||||||||||||
| α (.46%) | Np | ||||||||||||||||||
| SF ? | (various) | ||||||||||||||||||
| Am | 2200(80) keV | 14.0(10) ms | SF | (various) | (2+, 3−) | ||||||||||||||
| IT ? | Am | ||||||||||||||||||
| Am | 95 | 148 | 243.0613799(15) | 7,350(9) y | α | Np | 5/2− | ||||||||||||
| SF (3.7×10%) | (various) | ||||||||||||||||||
| Am | 2300(200) keV | 5.5(5) μs | SF | (various) | |||||||||||||||
| IT ? | Am | ||||||||||||||||||
| Am | 95 | 149 | 244.0642829(16) | 10.01(3) h | β | Cm | (6−) | ||||||||||||
| Am | 89.3(16) keV | 26.13(43) min | β (99.96%) | Cm | 1+ | ||||||||||||||
| EC (0.0364%) | Pu | ||||||||||||||||||
| Am | 2000(200)# | 900(150) μs | SF | (various) | |||||||||||||||
| IT ? | Am | ||||||||||||||||||
| Am | 2200(200)# | ~6.5 μs | SF | (various) | |||||||||||||||
| IT ? | Am | ||||||||||||||||||
| Am | 95 | 150 | 245.0664528(20) | 2.05(1) h | β | Cm | 5/2+ | ||||||||||||
| Am | 2400(400)# | 640(60) ns | SF | (various) | |||||||||||||||
| IT ? | Am | ||||||||||||||||||
| Am | 95 | 151 | 246.069774(19)# | 39(3) min | β | Cm | (7−) | ||||||||||||
| Am | 30(10)# keV | 25.0(2) min | β | Cm | 2(−) | ||||||||||||||
| IT ? | Am | ||||||||||||||||||
| Am | 2000(800)# keV | 73(10) μs | SF | (various) | |||||||||||||||
| IT ? | Am | ||||||||||||||||||
| Am | 95 | 152 | 247.07209(11)# | 23.0(13) min | β | Cm | 5/2# | ||||||||||||
| This table header & footer: | |||||||||||||||||||
- Am – Excited nuclear isomer.
- ( ) – Uncertainty (1σ) is given in concise form in parentheses after the corresponding last digits.
- # – Atomic mass marked #: value and uncertainty derived not from purely experimental data, but at least partly from trends from the Mass Surface (TMS).
-
Modes of decay:
CD: Cluster decay EC: Electron capture IT: Isomeric transition SF: Spontaneous fission - ( ) spin value – Indicates spin with weak assignment arguments.
- ^ # – Values marked # are not purely derived from experimental data, but at least partly from trends of neighboring nuclides (TNN).
- The discovery of this isotope is uncertain due to disagreements between theoretical predictions and reported experimental data.
Actinides vs fission products
| Actinides and fission products by half-life | ||||||||
|---|---|---|---|---|---|---|---|---|
| Actinides by decay chain | Half-life range (a) |
Fission products of U by yield | ||||||
| 4n | 4n + 1 | 4n + 2 | 4n + 3 | 4.5–7% | 0.04–1.25% | <0.001% | ||
| Ra | 4–6 a | Eu | ||||||
| Bk | > 9 a | |||||||
| Cm | Pu | Cf | Ac | 10–29 a | Sr | Kr | Cd | |
| U | Pu | Cm | 29–97 a | Cs | Sm | Sn | ||
| Cf | Am | 141–351 a |
No fission products have a half-life | |||||
| Am | Cf | 430–900 a | ||||||
| Ra | Bk | 1.3–1.6 ka | ||||||
| Pu | Th | Cm | Am | 4.7–7.4 ka | ||||
| Cm | Cm | 8.3–8.5 ka | ||||||
| Pu | 24.1 ka | |||||||
| Th | Pa | 32–76 ka | ||||||
| Np | U | U | 150–250 ka | Tc | Sn | |||
| Cm | Pu | 327–375 ka | Se | |||||
| 1.33 Ma | Cs | |||||||
| Np | 1.61–6.5 Ma | Zr | Pd | |||||
| U | Cm | 15–24 Ma | I | |||||
| Pu | 80 Ma |
... nor beyond 15.7 Ma | ||||||
| Th | U | U | 0.7–14.1 Ga | |||||
| ||||||||
Notable isotopes
Americium-241

Americium-241 is the most common isotope of americium in nuclear waste. It is the isotope used in an americium smoke detector based on an ionization chamber. It is a potential fuel for long-lifetime radioisotope thermoelectric generators.
| Parameter | Value |
|---|---|
| Atomic mass | 241.056829 u |
| Mass excess | 52930 keV |
| Beta decay energy | −767 keV |
| Spin | 5/2− |
| Half-life | 432.6 years |
| Spontaneous fissions | 1200 per kg s |
| Decay heat | 114 watts/kg |
Possible parent nuclides: beta from Pu, electron capture from Cm, alpha from Bk.
Am alpha decays, with a by-product of gamma rays. Its presence in plutonium is determined by the original concentration of Pu and the sample age. Due to the low penetration of alpha radiation, Am only poses a health risk when ingested or inhaled. Older samples of plutonium containing plutonium-241 contain a buildup of Am. A chemical removal of americium from reworked plutonium (e.g. during reworking of plutonium pits) may be required.
Americium-242m
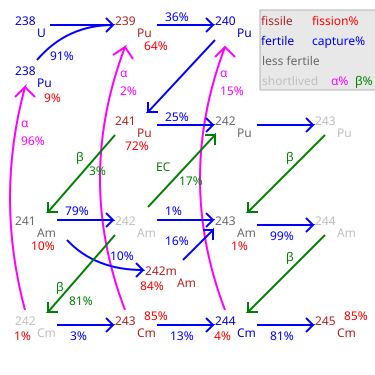
Fission percentage is 100 minus shown percentages.
Total rate of transmutation varies greatly by nuclide.
Cm–Cm are long-lived with negligible decay.
| Probability | Decay mode | Decay energy | Decay product |
|---|---|---|---|
| 99.54% | isomeric transition | 0.05 MeV | Am |
| 0.46% | alpha decay | 5.64 MeV | Np |
| (1.5±0.6) × 10 | spontaneous fission | ~200 MeV | fission products |
Americium-242m has a mass of 242.0595492 g/mol. It is one of the rare cases, like Ag, Ho, Ta, Re, Ir, Bi, Po and others, where a higher-energy nuclear isomer is more stable than the ground state, americium-242.
Am is fissile with a low critical mass, comparable to that of Pu. It has a very high fission cross section, and is quickly destroyed if it is produced in a nuclear reactor. It has been investigated whether this isotope could be used for a novel type of nuclear rocket.
| Probability | Decay mode | Decay energy | Decay product |
|---|---|---|---|
| 82.70% | beta decay | 0.665 MeV | Cm |
| 17.30% | electron capture | 0.751 MeV | Pu |
Americium-243

Americium-243 has a mass of 243.06138 g/mol and a half-life of 7,370 years, the longest lasting of all americium isotopes. It is formed in the nuclear fuel cycle by neutron capture on plutonium-242 followed by beta decay. Production increases exponentially with increasing burnup as a total of 5 neutron captures on U are required. If MOX-fuel is used, particularly MOX-fuel high in
Pu and
Pu, more americium overall and more
Am will be produced.
It decays by either emitting an alpha particle (with a decay energy of 5.27 MeV) to become Np, which then quickly decays to Pu, or rarely, by spontaneous fission.
As for the other americium isotopes, and more generally for all alpha emitters, Am is carcinogenic in case of internal contamination after being inhaled or ingested. Am also presents a risk of external irradiation associated with the gamma ray emitted by its short-lived decay product Np. The external irradiation risk for the other two americium isotopes (Am and Am) is less than 10% of that for americium-243.
References
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- Sun, M. D.; et al. (2017). "New short-lived isotope Np and the absence of the Z = 92 subshell closure near N = 126". Physics Letters B. 771: 303–308. Bibcode:2017PhLB..771..303S. doi:10.1016/j.physletb.2017.03.074.
- Plus radium (element 88). While actually a sub-actinide, it immediately precedes actinium (89) and follows a three-element gap of instability after polonium (84) where no nuclides have half-lives of at least four years (the longest-lived nuclide in the gap is radon-222 with a half life of less than four days). Radium's longest lived isotope, at 1,600 years, thus merits the element's inclusion here.
- Specifically from thermal neutron fission of uranium-235, e.g. in a typical nuclear reactor.
- Milsted, J.; Friedman, A. M.; Stevens, C. M. (1965). "The alpha half-life of berkelium-247; a new long-lived isomer of berkelium-248". Nuclear Physics. 71 (2): 299. Bibcode:1965NucPh..71..299M. doi:10.1016/0029-5582(65)90719-4.
"The isotopic analyses disclosed a species of mass 248 in constant abundance in three samples analysed over a period of about 10 months. This was ascribed to an isomer of Bk with a half-life greater than 9 . No growth of Cf was detected, and a lower limit for the β half-life can be set at about 10 . No alpha activity attributable to the new isomer has been detected; the alpha half-life is probably greater than 300 ." - This is the heaviest nuclide with a half-life of at least four years before the "sea of instability".
- Excluding those "classically stable" nuclides with half-lives significantly in excess of Th; e.g., while Cd has a half-life of only fourteen years, that of Cd is eight quadrillion years.
- ^ "Americium" Archived 2012-07-30 at the Wayback Machine. Argonne National Laboratory, EVS. Retrieved 25 December 2009.
- Sasahara, Akihiro; Matsumura, Tetsuo; Nicolaou, Giorgos; Papaioannou, Dimitri (April 2004). "Neutron and Gamma Ray Source Evaluation of LWR High Burn-up UO2 and MOX Spent Fuels". Journal of Nuclear Science and Technology. 41 (4): 448–456. doi:10.3327/jnst.41.448.
- J. T. Caldwell; S. C. Fultz; C. D. Bowman; R. W. Hoff (March 1967). "Spontaneous Fission Half-Life of Am". Physical Review. 155 (4): 1309–1313. Bibcode:1967PhRv..155.1309C. doi:10.1103/PhysRev.155.1309. (halflife (9.5±3.5)×10 years)
- 95-Am-242 Archived 2011-07-19 at the Wayback Machine
- "Critical Mass Calculations for Am, Am and Am" (PDF). Archived from the original (PDF) on July 22, 2011. Retrieved February 3, 2011.
- "Extremely Efficient Nuclear Fuel Could Take Man To Mars In Just Two Weeks" (Press release). Ben-Gurion University Of The Negev. December 28, 2000.
- Ronen, Yigal; Shwageraus, E. (2000). "Ultra-thin 241mAm fuel elements in nuclear reactors". Nuclear Instruments and Methods in Physics Research A. 455 (2): 442–451. Bibcode:2000NIMPA.455..442R. doi:10.1016/s0168-9002(00)00506-4.
- ^ "Americium-243" Archived 2011-02-25 at the Wayback Machine. Oak Ridge National Laboratory. Retrieved 25 December 2009.
- "Isotopes of the Element Americium". Jefferson Lab Science Education. Retrieved 25 December 2009.
Sources
- Isotope masses from:
- Half-life, spin, and isomer data selected from the following sources.
- National Nuclear Data Center. "NuDat 2.x database". Brookhaven National Laboratory.
- IAEA - Nuclear Data Section. Live Chart of Nuclides. Vienna International Centre.
- Holden, Norman E. (2004). "11. Table of the Isotopes". In Lide, David R. (ed.). CRC Handbook of Chemistry and Physics (85th ed.). Boca Raton, Florida: CRC Press. ISBN 978-0-8493-0485-9.