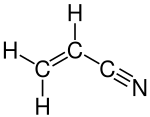
In organic chemistry, ammoxidation is a process for the production of nitriles (R−C≡N) using ammonia (NH3) and oxygen (O2). It is sometimes called the SOHIO process, acknowledging that ammoxidation was developed at Standard Oil of Ohio. The usual substrates are alkenes. Several million tons of acrylonitrile are produced in this way annually:
Scope
Ammoxidation of alkenes exploits the weak C-H bonds that are located in the allylic position of unsaturated hydrocarbons. Benzylic C-H bonds are also susceptible to ammoxidation, reflecting the weakness of their C-H bonds. Benzonitrile is produced from toluene, and phthalonitriles are produced from xylenes. The reaction represents a partial oxidation. Many byproducts are generated, but the feedstocks are often simple, which compensates for these losses. Additionally, some byproducts are useful or recyclable. For the production of acrylonitrile, byproducts include hydrogen cyanide, acrolein, and the solvent acetonitrile.
The reaction tolerates heteroatoms and substituents. Cyanopyridines (e.g. 3-cyanopyridine, the precursor to niacin) is produced from methylpyridines. 2- and 4-Chlorotoluene are converted to 2-chlorobenzonitrile and 4-chlorobenzonitrile, respectively.
Typical catalysts are the oxides of vanadium and molybdenum. The original catalyst discovered at Sohio was bismuth phosphomolybdate (BiPMo12O40). π-Allyl complexes are assumed as intermediates.
Related processes
Instead of alkenes, alcohols and aldehydes are competent substrates:
These substrates are usually more expensive than the alkenes, so they are less common. The nitrile process is used industrially to produce nitriles from fatty acids:
Hydrogen cyanide is prepared by an ammoxidation-like reaction of methane, the Andrussov oxidation:
See also
- Hydroamination - addition of amines to alkenes
References
- ^ Pollak, Peter; Romeder, Gérard; Hagedorn, Ferdinand; Gelbke, Heinz-Peter (2000). "Nitriles". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_363. ISBN 3527306730.
- "Sohio Acrylonitrile Process - American Chemical Society". American Chemical Society. Retrieved 11 July 2017.
- Peter Pollak, Gérard Romeder, Ferdinand Hagedorn, Heinz-Peter Gelbke "Nitriles" Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a17_363
- "The Sohio Acrylonitrile Process". National Historic Chemical Landmarks. American Chemical Society. Archived from the original on February 23, 2013. Retrieved March 25, 2013.
- ^ Pollak, Peter; Romeder, Gérard; Hagedorn, Ferdinand; Gelbke, Heinz-Peter (2000). "Nitriles". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_363. ISBN 3527306730.
- Nugent, W. A.; Mayer, J. M., Metal-Ligand Multiple Bonds. J. Wiley: New York, 1988.




