| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Oxepine | |||
| Other names Oxacycloheptatriene | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C6H6O | ||
| Molar mass | 94.113 g·mol | ||
| Related compounds | |||
| Related compounds | Cyclohexene oxide Oxonane | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Oxepin is an oxygen-containing heterocycle consisting of a seven-membered ring with three double bonds. The parent C6H6O exists as an equilibrium mixture with benzene oxide.
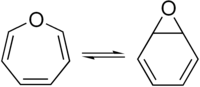
The oxepin–benzene oxide equilibrium is affected by the ring substituents. A related dimethyl derivative exists mainly as the oxepin isomer, an orange liquid.
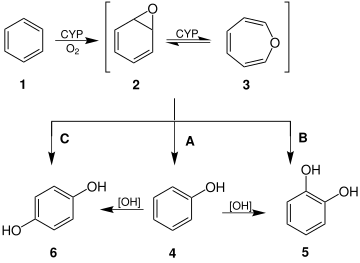
Oxepin is an intermediate in the oxidation of benzene by the cytochrome P450 (CYP). Other arene oxides are metabolites of the parent arene.
References
- Vogel, E.; Günther, H. (1967). "Benzene Oxide–Oxepin Valence Tautomerism". Angewandte Chemie International Edition in English. 6 (5): 385–401. doi:10.1002/anie.196703851.
- Paquette, Leo A.; Barrett, J. H. (1969). "2,7-Dimethyloxepin". Org. Synth. 49: 62. doi:10.15227/orgsyn.049.0062.
- Snyder, R.; Witz, G.; Goldstein, B. D. (1993). "The Toxicology of Benzene". Environmental Health Perspectives. 100: 293–306. doi:10.1289/ehp.93100293. JSTOR 3431535. PMC 1519582. PMID 8354177.