| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Isotopes of boron" – news · newspapers · books · scholar · JSTOR (May 2018) (Learn how and when to remove this message) |
| ||||||||||||||||||||||||||
| Standard atomic weight Ar°(B) | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||
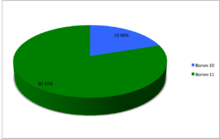
Boron (5B) naturally occurs as isotopes
B
and
B
, the latter of which makes up about 80% of natural boron. There are 13 radioisotopes that have been discovered, with mass numbers from 7 to 21, all with short half-lives, the longest being that of
B
, with a half-life of only 771.9(9) ms and
B
with a half-life of 20.20(2) ms. All other isotopes have half-lives shorter than 17.35 ms. Those isotopes with mass below 10 decay into helium (via short-lived isotopes of beryllium for
B
and
B
) while those with mass above 11 mostly become carbon.
List of isotopes
| Nuclide |
Z | N | Isotopic mass (Da) |
Half-life |
Decay mode |
Daughter isotope |
Spin and parity |
Natural abundance (mole fraction) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excitation energy | Normal proportion | Range of variation | |||||||||||||||||
B ? |
5 | 1 | 6.050800(2150) | p-unstable | 2p? | Li ? |
2−# | ||||||||||||
B |
5 | 2 | 7.029712(27) | 570(14) ys |
p | Be |
(3/2−) | ||||||||||||
B |
5 | 3 | 8.0246073(11) | 771.9(9) ms | βα | He |
2+ | ||||||||||||
B |
10624(8) keV | 0+ | |||||||||||||||||
B |
5 | 4 | 9.0133296(10) | 800(300) zs | p | Be |
3/2− | ||||||||||||
B |
5 | 5 | 10.012936862(16) | Stable | 3+ | ||||||||||||||
B |
5 | 6 | 11.009305167(13) | Stable | 3/2− | ||||||||||||||
B |
12560(9) keV | 1/2+, (3/2+) | |||||||||||||||||
B |
5 | 7 | 12.0143526(14) | 20.20(2) ms | β (99.40(2)%) | C |
1+ | ||||||||||||
| βα (0.60(2)%) | Be | ||||||||||||||||||
B |
5 | 8 | 13.0177800(11) | 17.16(18) ms | β (99.734(36)%) | C |
3/2− | ||||||||||||
| βn (0.266(36)%) | C | ||||||||||||||||||
B |
5 | 9 | 14.025404(23) | 12.36(29) ms | β (93.96(23)%) | C |
2− | ||||||||||||
| βn (6.04(23)%) | C | ||||||||||||||||||
| β2n ? | C ? | ||||||||||||||||||
B |
17065(29) keV | 4.15(1.90) zs | IT ? | 0+ | |||||||||||||||
B |
5 | 10 | 15.031087(23) | 10.18(35) ms | βn (98.7(1.0)%) | C |
3/2− | ||||||||||||
| β (< 1.3%) | C | ||||||||||||||||||
| β2n (< 1.5%) | C | ||||||||||||||||||
B |
5 | 11 | 16.039841(26) | > 4.6 zs | n ? | B ? |
0− | ||||||||||||
B |
5 | 12 | 17.04693(22) | 5.08(5) ms | βn (63(1)%) | C |
(3/2−) | ||||||||||||
| β (21.1(2.4)%) | C | ||||||||||||||||||
| β2n (12(2)%) | C | ||||||||||||||||||
| β3n (3.5(7)%) | C | ||||||||||||||||||
| β4n (0.4(3)%) | C | ||||||||||||||||||
B |
5 | 13 | 18.05560(22) | < 26 ns | n | B |
(2−) | ||||||||||||
B |
5 | 14 | 19.06417(56) | 2.92(13) ms | βn (71(9)%) | C |
(3/2−) | ||||||||||||
| β2n (17(5)%) | C | ||||||||||||||||||
| β3n (< 9.1%) | C | ||||||||||||||||||
| β (> 2.9%) | C | ||||||||||||||||||
B |
5 | 15 | 20.07451(59) | > 912.4 ys | n | B |
(1−, 2−) | ||||||||||||
B |
5 | 16 | 21.08415(60) | > 760 ys | 2n | B |
(3/2−) | ||||||||||||
| This table header & footer: | |||||||||||||||||||
- B – Excited nuclear isomer.
- ( ) – Uncertainty (1σ) is given in concise form in parentheses after the corresponding last digits.
- # – Atomic mass marked #: value and uncertainty derived not from purely experimental data, but at least partly from trends from the Mass Surface (TMS).
-
Modes of decay:
n: Neutron emission p: Proton emission - Bold symbol as daughter – Daughter product is stable.
- ( ) spin value – Indicates spin with weak assignment arguments.
- # – Values marked # are not purely derived from experimental data, but at least partly from trends of neighboring nuclides (TNN).
- This isotope has not yet been observed; given data is inferred or estimated from periodic trends.
- Subsequently decays by double proton emission to
He
for a net reaction of
B
→
He
+ 3
H
- Has 1 halo proton
- Intermediate product of a branch of proton-proton chain in stellar nucleosynthesis as part of the process converting hydrogen to helium
- Immediately decays into two α particles, for a net reaction of
B
→ 2
He
+
H
- One of the few stable odd-odd nuclei
- Immediately decays into two α particles, for a net reaction of
B
→ 3
He
+ e - ^ Decay mode shown is energetically allowed, but has not been experimentally observed to occur in this nuclide.
- ^ Has 2 halo neutrons
Boron-8
Boron-8 is an isotope of boron that undergoes β decay to beryllium-8 with a half-life of 771.9(9) ms. It is the strongest candidate for a halo nucleus with a loosely-bound proton, in contrast to neutron halo nuclei such as lithium-11.
Although neutrinos from boron-8 beta decays within the Sun make up only about 80 ppm of the total solar neutrino flux, they have a higher energy centered around 10 MeV, and are an important background to dark matter direct detection experiments. They are the first component of the neutrino floor that dark matter direct detection experiments are expected to eventually encounter.
Applications
Boron-10
Boron-10 is used in boron neutron capture therapy as an experimental treatment of some brain cancers.
References
- "Standard Atomic Weights: Boron". CIAAW. 2009.
- Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- Wang, Meng; Huang, W.J.; Kondev, F.G.; Audi, G.; Naimi, S. (2021). "The AME 2020 atomic mass evaluation (II). Tables, graphs and references*". Chinese Physics C. 45 (3): 030003. doi:10.1088/1674-1137/abddaf.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ "Atomic Weight of Boron". CIAAW.
- ^ Leblond, S.; et al. (2018). "First observation of B and B". Physical Review Letters. 121 (26): 262502–1–262502–6. arXiv:1901.00455. doi:10.1103/PhysRevLett.121.262502. PMID 30636115. S2CID 58602601.
- Maaß, Bernhard; Müller, Peter; Nörtershäuser, Wilfried; Clark, Jason; Gorges, Christian; Kaufmann, Simon; König, Kristian; Krämer, Jörg; Levand, Anthony; Orford, Rodney; Sánchez, Rodolfo; Savard, Guy; Sommer, Felix (November 2017). "Towards laser spectroscopy of the proton-halo candidate boron-8". Hyperfine Interactions. 238 (1): 25. Bibcode:2017HyInt.238...25M. doi:10.1007/s10751-017-1399-5. S2CID 254551036.
- Bellerive, A. (2004). "Review of solar neutrino experiments". International Journal of Modern Physics A. 19 (8): 1167–1179. arXiv:hep-ex/0312045. Bibcode:2004IJMPA..19.1167B. doi:10.1142/S0217751X04019093. S2CID 16980300.
- Cerdeno, David G.; Fairbairn, Malcolm; Jubb, Thomas; Machado, Pedro; Vincent, Aaron C.; Boehm, Celine (2016). "Physics from solar neutrinos in dark matter direct detection experiments". JHEP. 2016 (5): 118. arXiv:1604.01025. Bibcode:2016JHEP...05..118C. doi:10.1007/JHEP05(2016)118. S2CID 55112052.