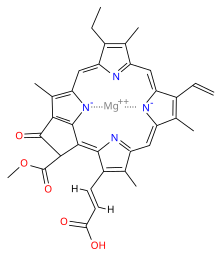
Chlorophyll c refers to forms of chlorophyll found in certain marine algae, including the photosynthetic Chromista (e.g. diatoms and brown algae) and dinoflagellates. These pigments are characterized by their unusual chemical structure, with a porphyrin as opposed to the chlorin (which has a reduced ring D) as the core; they also do not have an isoprenoid tail. Both these features stand out from the other chlorophylls commonly found in algae and plants.
It has a blue-green color and is an accessory pigment, particularly significant in its absorption of light in the 447–520 nm wavelength region. Like chlorophyll a and chlorophyll b, it helps the organism gather light and passes a quanta of excitation energy through the light harvesting antennae to the photosynthetic reaction centre.
Chlorophyll c can be further divided into chlorophyll c1, chlorophyll c2, and chlorophyll c3, plus at least eight other more recently found subtypes.
Chlorophyll c1
Chlorophyll c1 is a common form of chlorophyll c. It differs from chlorophyll c2 in its C8 group, having an ethyl group instead of vinyl group (C-C single bond instead of C=C double bond). Its absorption maxima are around 444, 577, 626 nm and 447, 579, 629 nm in diethyl ether and acetone respectively.
Chlorophyll c2
Chlorophyll c2 is the most common form of chlorophyll c. Its absorption maxima are around 447, 580, 627 nm and 450, 581, 629 nm in diethyl ether and acetone respectively.
Chlorophyll c3
Chlorophyll c3 is a form of chlorophyll c found in microalga Emiliania huxleyi, identified in 1989. Its absorption maxima are around 452, 585, 625 nm and 452, 585, 627 nm in diethyl ether and acetone respectively.
Biosynthesis
Chlorophyll c synthesis branches off early from the typical Chlorophyllide synthesis pathway, after divinylprotochlorophyllide (DV-PChlide) is formed. It has been established that DV-PChlide and MV-PChlide are processed directly by a 17 oxidase (CHLC, chlorophyll c synthase) into Chl c2 and Chl c1, respectively. The 17 oxidtion was proposed to proceed by "hydroxylation of the 17-propionate reside at the 17-position and successive dehydration to the 17-acrylate residue." An 8-vinyl reductase (elaborating on the promiscuous behavior of ferredoxin-type 3,8-divinyl chlorophyllide reductase) could also convert Chl c2 into Chl c1. The two steps could be swapped for the same effect.
Structure
| Chlorophyll c1 | Chlorophyll c2 | Chlorophyll c3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
- Speer, B. R. "Photosynthetic Pigments". Retrieved 2 August 2014.
- ^ Blankenship RE (February 2002). Molecular Mechanisms of Photosynthesis. Wiley-Blackwell.
- ^ Dougherty RC, Strain HH, Svec WA, Uphaus RA, Katz JJ (May 1970). "The structure, properties, and distribution of chlorophyll c". Journal of the American Chemical Society. 92 (9): 2826–33. Bibcode:1970JAChS..92.2826D. doi:10.1021/ja00712a037. PMID 5439971.
- ^ Fookes CJ, Jeffrey SW (1989). "The structure of chlorophyll c3, a novel marine photosynthetic pigment". J. Chem. Soc., Chem. Commun. (23): 1827–28. doi:10.1039/C39890001827.
- Zapata M, Garrido JL, Jeffrey SW (2006). "Chlorophyll c Pigments: Current Status". Chlorophylls and Bacteriochlorophylls: Advances in Photosynthesis and Respiration. Advances in Photosynthesis and Respiration. 25: 39–53. doi:10.1007/1-4020-4516-6_3. ISBN 978-1-4020-4515-8.
- ^ Fawley MW (October 1989). "A new form of chlorophyll C involved in light-harvesting". Plant Physiology. 91 (2): 727–32. doi:10.1104/pp.91.2.727. PMC 1062062. PMID 16667093.
- Jeffrey SW (September 1976). "The Occurrence of Chlorophyll c1 and c2 in Algae". Journal of Phycology. 12 (3): 349–354. Bibcode:1976JPcgy..12..349J. doi:10.1111/j.1529-8817.1976.tb02855.x. S2CID 83927313.
- ^ Jiang, Yanyou; Cao, Tianjun; Yang, Yuqing; Zhang, Huan; Zhang, Jingyu; Li, Xiaobo (2023-10-06). "A chlorophyll c synthase widely co-opted by phytoplankton". Science. 382 (6666): 92–98. doi:10.1126/science.adg7921.
- Xu, M; Kinoshita, Y; Matsubara, S; Tamiaki, H (March 2016). "Synthesis of chlorophyll-c derivatives by modifying natural chlorophyll-a". Photosynthesis Research. 127 (3): 335–45. Bibcode:2016PhoRe.127..335X. doi:10.1007/s11120-015-0190-1. PMID 26346903. S2CID 254944200.
- Ito, Hisashi; Tanaka, Ayumi (March 2014). "Evolution of a New Chlorophyll Metabolic Pathway Driven by the Dynamic Changes in Enzyme Promiscuous Activity". Plant and Cell Physiology. 55 (3): 593–603. doi:10.1093/pcp/pct203. hdl:2115/58225. PMID 24399236.
| Types of tetrapyrroles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Bilanes (Linear) |
| ||||||||
| Macrocycle | |||||||||
| Types of plant pigments | |
|---|---|
| Betalains | |
| Chlorophyll | |
| Curcuminoids | |
| Flavonoids | |
| Carotenoids | |
| Other | |