| |||
| |||
| Identifiers | |||
|---|---|---|---|
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | F2H2Si | ||
| Molar mass | 68.098 g·mol | ||
| Appearance | colourless gas | ||
| Melting point | −122 °C (−188 °F; 151 K) | ||
| Boiling point | −77.8 °C (−108.0 °F; 195.3 K) | ||
| Thermochemistry | |||
| Std molar entropy (S298) |
262.12 J/mol•K | ||
| Std enthalpy of formation (ΔfH298) |
-790.78 kJ/mol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |||
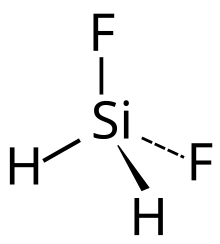
Difluorosilane is a gaseous chemical compound with formula SiH2F2. It can be considered as a derivative of silane with two hydrogen atoms replaced with fluorine.
Production
Difluorosilane can be made by fluorinating dichlorosilane with antimony trifluoride.
- 3 SiH2Cl2 + 2 SbF3 → 3 SiH2F2 + 2 SbCl3
Some is also made in a reaction of silicon tetrafluoride with hydrogen
- SiF4 + 2 H2 → SiH2F2 + 2 HF
Traces of difluorosilane are made when coal is burnt.
Properties
Difluorosilane is a gas with boiling point −77.8 °C, and a freezing point of −122 °C. It has no colour. The silicon–fluorine bond length in difluorosilane is 1.358 Å which is greater than that in fluorosilane but less than the length in trifluorosilane.
Reactions
In an electric discharge, hydrogen atoms are preferentially removed from the molecule and SiHF2SiHF2 is formed along with hydrogen.
- 2 SiH2F2 → SiHF2SiHF2 + H2
At elevated temperatures, difluorosilane can disproportionate by swapping hydrogen and fluorine atoms between molecules to form fluorosilane and trifluorosilane.
Use
Difluorosilane is used in dental varnish in order to prevent tooth cavities.
Difluorosilane is also used in chemical vapour deposition to deposit silicon nitride films.
References
- Chase, M. W. (1998). "NIST-JANAF Themochemical Tables, Fourth Edition": 1–1951.
{{cite journal}}: Cite journal requires|journal=(help) - 郝润蓉 等. 无机化学丛书 第三卷 碳 硅 锗分族. 科学出版社, 1998. pp 178. 2.3 卤代硅烷
- ^ Addison, C. C. (1973). Inorganic Chemistry of the Main-Group Elements. Royal Society of Chemistry. p. 188. ISBN 9780851867526.
- Kruszewski, Łukasz; Fabiańska, Monika J.; Ciesielczuk, Justyna; Segit, Tomasz; Orłowski, Ryszard; Motyliński, Rafał; Kusy, Danuta; Moszumańska, Izabela (November 2018). "First multi-tool exploration of a gas-condensate-pyrolysate system from the environment of burning coal mine heaps: An in situ FTIR and laboratory GC and PXRD study based on Upper Silesian materials". Science of the Total Environment. 640–641: 1044–1071. Bibcode:2018ScTEn.640.1044K. doi:10.1016/j.scitotenv.2018.05.319. PMID 30021271. S2CID 51703425.
- ^ Ebsworth, E. A. V. (2013). Volatile Silicon Compounds: International Series of Monographs on Inorganic Chemistry. Elsevier. pp. 54–56. ISBN 9781483180557.
- Brambilla, Eugenio (2001). "Fluoride – Is It Capable of Fighting Old and New Dental Diseases?". Caries Research. 35 (1): 6–9. doi:10.1159/000049101. PMID 11359049. S2CID 24969435.

