| |
| Names | |
|---|---|
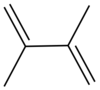
| Preferred IUPAC name 2,3-Dimethylbuta-1,3-diene | |
| Other names Biisopropenyl; Diisopropenyl; 2,3-Dimethylbutadiene; 2,3-Dimethylenebutane | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.007.430 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H10 |
| Molar mass | 82.146 g·mol |
| Density | 0.7222g / cm |
| Melting point | −76 °C (−105 °F; 197 K) |
| Boiling point | 69 °C (156 °F; 342 K) |
| Vapor pressure | 269 mm Hg (37.7 °C) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Flammable and irritant |
| GHS labelling: | |
| Pictograms | 
|
| Flash point | −1 °C (30 °F; 272 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Dimethylbutadiene, formally referred to as 2,3-dimethyl-1,3-butadiene, is an organic compound with the formula (CH3)2C4H4. It is colorless liquid which served an important role in the early history of synthetic rubber. It is now a specialty reagent.
Synthesis
Dimethylbutadiene is readily prepared by an acid catalyzed dehydration reaction of pinacol:
- HO(CH3)2C−C(CH3)2OH → CH3(CH2=C−C=CH2)CH3 + 2 H2O
The current industrial route involves dimerization of propene followed by dehydrogenation.
Applications
In 1909, Fritz Hofmann and a team working at Bayer succeeded in polymerizing dimethylbutadiene. It was then called methyl isoprene because it has one more methyl group than isoprene. Their polymer was the first synthetic rubber. The polymer had a number of deficiencies relative to natural rubber. The Bayer synthesis of dimethylbutadiene involved the dehydration of pinacol, as described above.
Reactions
Dimethylbutadiene readily undergoes Diels-Alder reactions and reacts faster than 1,3-butadiene. Its effectiveness in this reaction is attributed to the stabilization of the cis-conformation owing to the influence of the methyl groups on the C2 and C3 positions.

References
- Haynes, W. M.; Lide, D. R. (2012). CRC Handbook of Chemistry and Physics 93rd Ed. CRC Press/Taylor and Francis. ISBN 978-1439880494.
- "CSID:10124". Retrieved 19 October 2012.
- C. F. H. Allen, Alan Bell, L. W. Newton, and E. R. Coburn (1942). "2,3-Dimethyl-1,3-butadiene". Organic Syntheses. 22: 39
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 3, p. 312. - ^ Griesbaum, Karl; Behr, Arno; Biedenkapp, Dieter; Voges, Heinz-Werner; Garbe, Dorothea; Paetz, Christian; Collin, Gerd; Mayer, Dieter; Höke, Hartmut (2000), "Hydrocarbons", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a13_227, ISBN 3527306730.
- The Moving Powers of Rubber, Leverkusen, Germany: LANXESS AG: 20.
- "A Poor Substitute". Retrieved 18 October 2012.