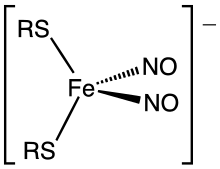
In biochemistry, dinitrosyl iron complexes (DNIC's) are coordination complexes with the formula . Together with Roussin esters (Fe2(NO)4(SR)2), they result from the degradation of iron-sulfur proteins by nitric oxide. Commonly the thiolate ligands are cysteinyl residues or glutathione. These metal nitrosyl complexes have attracted much attention because they serve as biochemical signals in response to oxidative stress, manifested in the formation of NO. The anions are tetrahedral.
References
- Crack, J. C.; Green, J.; Thomson, A. J.; Brun, N. E. L. (2014). "Iron–Sulfur Clusters as Biological Sensors: The Chemistry of Reactions with Molecular Oxygen and Nitric Oxide". Accounts of Chemical Research. 47 (10): 3196–3205. doi:10.1021/ar5002507. PMID 25262769.
- Jessica Fitzpatrick; Eunsuk Kim (2015). "Synthetic Modeling Chemistry of Iron–Sulfur Clusters in Nitric Oxide Signaling". Acc. Chem. Res. 48 (8): 2453–2461. doi:10.1021/acs.accounts.5b00246. PMID 26197209.