| |||
| Names | |||
|---|---|---|---|
| IUPAC name Dithionous acid | |||
| Other names Hydrosulfurous acid; Hyposulfurous acid | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
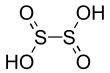
| Chemical formula | H2S2O4 | ||
| Molar mass | 130.144 g/mol | ||
| Acidity (pKa) | 0.35, 2.45 | ||
| Conjugate base | Dithionite | ||
| Related compounds | |||
| Related compounds | Oxalic acid Sodium dithionite Potassium dithionite | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Dithionous acid is a sulfur oxoacid with the chemical formula H2S2O4. It has not been observed experimentally. It is the conjugate base of the dithionite dianion, S2O4. Dithionite is a well-known reducing agent.
Related compounds
- Dithionic acid (H2S2O6), another unstable protonated sulfur oxide, derived from dithionate.
References
- Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 16: The group 16 elements". Inorganic Chemistry, 3rd Edition. Pearson. p. 520. ISBN 978-0-13-175553-6.
- Drozdova, Yana; Steudel, Ralf; Hertwig, Roland H.; Koch, Wolfram; Steiger, Thomas (1998). "Structures and Energies of Various Isomers of Dithionous Acid, H2S2O4, and of Its Anion HS2O4- 1". The Journal of Physical Chemistry A. 102 (6): 990–996. Bibcode:1998JPCA..102..990D. doi:10.1021/jp972658d.
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |