| |
| Names | |
|---|---|
| Preferred IUPAC name Dodecanedioic acid | |
| Other names DDDA | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.010.680 |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
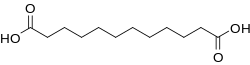
| Chemical formula | C12H22O4 |
| Molar mass | 230.304 g·mol |
| Appearance | White flakes |
| Density | 1.066 g/cm |
| Melting point | 127–129 °C (261–264 °F; 400–402 K) |
| Boiling point | 245 °C (473 °F; 518 K) |
| Solubility in water | pH dependent |
| Hazards | |
| Flash point | 220 °C (428 °F; 493 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Dodecanedioic acid (DDDA) is a dicarboxylic acid with the formula (CH2)10(CO2H)2. A white solid, the compound finds a variety of applications ranging from polymers to materials. The unbranched compound is the most commonly encountered C12 dicarboxylic acid.
Production
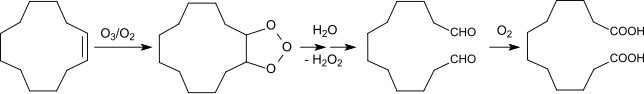
DDDA has traditionally been produced from butadiene using a multi-step chemical process. Butadiene is first converted to cyclododecatriene through cyclotrimerization. The triene is then hydrogenated to cyclododecane. Autoxidation by air in the presence of boric acid gives a mixture of cyclodecanol and the cyclododecanone. In the final step, this mixture oxidized to the diacid using nitric acid. An alternative route involves ozonolysis of cyclododecene.
Biological process
Paraffin wax can be converted into DDDA on a laboratory scale with a special strain of Candida tropicalis yeast in a multi-step process. Renewable plant-oil feedstocks sourced from switchgrass could also be used to produce DDDA.
Uses
DDDA is used in antiseptics, top-grade coatings, painting materials, corrosion inhibitors, surfactants, and polymers. It is one of two precursors to the engineering plastic nylon 612. The once commercial nylon called Qiana was produced on scale using DDDA. DDDA ester with ethylene glycol is a synthetic musk of the macrocyclic lactones group commercially marketed as "Arova 16".
Medical
In type 2 diabetic patients DDDA demonstrated that IV infusion helps to maintain normal blood sugar and energy levels without increasing the blood glucose load in the process.
References
- ^ "BIOLON® DDDA". verdezyne.com. Archived from the original on 2016-09-24. Retrieved 2016-09-23.
- Klaus Weissermel, Hans-Jurgen Arpe (1997). Industrial Organic Chemistry (3rd ed.). John Wiley & Sons. ISBN 3-527-28838-4.
- Cornils, Boy; Lappe, Peter; By Staff, Updated (2014). "Dicarboxylic Acids, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. pp. 1–18. doi:10.1002/14356007.a08_523.pub3. ISBN 9783527306732.
- "Dibasic acids". www.cathaybiotech.com. Archived from the original on 2018-10-09. Retrieved 2019-03-15.
- Kroha, Kyle. "Industrial biotechnology provides opportunities for commercial production of new long-chain dibasic acids" (PDF). Inform. 15(9) (Sep 2004). American Oil Chemists Society: 568. Archived from the original (PDF) on 6 October 2014. Retrieved 15 March 2019.
- Nylon#Homopolymers
- Greco, A. V.; Mingrone, G; Capristo, E; Benedetti, G; De Gaetano, A; Gasbarrini, G (1998). "The metabolic effect of dodecanedioic acid infusion in non-insulin-dependent diabetic patients". Nutrition. 14 (4): 351–7. doi:10.1016/s0899-9007(97)00502-9. PMID 9591306.
| Linear saturated dicarboxylic acids (HO2C-R-CO2H) | |
|---|---|
| |
| Category:Dicarboxylic acids |