 | |
| Clinical data | |
|---|---|
| Trade names | Ebernet |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
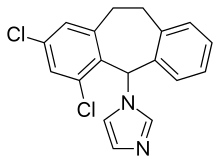
| Formula | C18H14Cl2N2 |
| Molar mass | 329.22 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Eberconazole is an antifungal drug. As a 1% topical cream, it is an effective treatment for dermatophytosis, candidiasis, and pityriasis.
It was approved for use in Spain in 2015 and is sold under the trade name Ebernet. It is also approved for use in Panama, Guatemala, Costa Rica, Honduras, and the Dominican Republic.
References
- ^ "Ebernet". NewBridge Pharmaceuticals. Archived from the original on 2016-04-24. Retrieved 2017-01-15.
- del Palacio A, Ortiz FJ, Pérez A, Pazos C, Garau M, Font E (2001). "A double-blind randomized comparative trial: eberconazole 1% cream versus clotrimazole 1% cream twice daily in Candida and dermatophyte skin infections". Mycoses. 44 (5): 173–80. doi:10.1046/j.1439-0507.2001.00632.x. PMID 11486455.
- Repiso Montero T, López S, Rodríguez C, del Rio R, Badell A, Gratacós MR (May 2006). "Eberconazole 1% cream is an effective and safe alternative for dermatophytosis treatment: multicenter, randomized, double-blind, comparative trial with miconazole 2% cream". International Journal of Dermatology. 45 (5): 600–4. doi:10.1111/j.1365-4632.2006.02841.x. PMID 16700802.
- "Eberconazole". Drugs.com. Archived from the original on 2021-01-17. Retrieved 2017-01-15.
This dermatologic drug article is a stub. You can help Misplaced Pages by expanding it. |