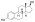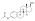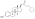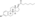| |
 | |
| Clinical data | |
|---|---|
| Other names | Estradiol 17β-phosphate; Estra-1,3,5(10)-triene-3,17β-diol 17β-(dihydrogen phosphate); 3-Hydroxyestra-1,3,5(10)-trien-17β-yl phosphate |
| Drug class | Estrogen; Estrogen ester |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H25O5P |
| Molar mass | 352.367 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Estradiol phosphate, or estradiol 17β-phosphate, also known as estra-1,3,5(10)-triene-3,17β-diol 17β-(dihydrogen phosphate), is an estrogen which was never marketed. It is an estrogen ester, specifically an ester of estradiol with phosphoric acid, and acts as a prodrug of estradiol in the body. It is rapidly cleaved by phosphatase enzymes into estradiol upon administration. Estradiol phosphate is contained within the chemical structures of two other estradiol esters, polyestradiol phosphate (a polymer of estradiol phosphate) and estramustine phosphate (estradiol 3-normustine 17β-phosphate), both of which have been marketed for the treatment of prostate cancer.
| Estrogen | Structure | Ester(s) | Relative mol. weight |
Relative E2 content |
log P | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Length | ||||||
| Estradiol | – | – | – | – | 1.00 | 1.00 | 4.0 | ||
| Estradiol acetate | C3 | Ethanoic acid | Straight-chain fatty acid | 2 | 1.15 | 0.87 | 4.2 | ||
| Estradiol benzoate | C3 | Benzoic acid | Aromatic fatty acid | – (~4–5) | 1.38 | 0.72 | 4.7 | ||
| Estradiol dipropionate | C3, C17β | Propanoic acid (×2) | Straight-chain fatty acid | 3 (×2) | 1.41 | 0.71 | 4.9 | ||
| Estradiol valerate | C17β | Pentanoic acid | Straight-chain fatty acid | 5 | 1.31 | 0.76 | 5.6–6.3 | ||
| Estradiol benzoate butyrate | C3, C17β | Benzoic acid, butyric acid | Mixed fatty acid | – (~6, 2) | 1.64 | 0.61 | 6.3 | ||
| Estradiol cypionate | C17β | Cyclopentylpropanoic acid | Cyclic fatty acid | – (~6) | 1.46 | 0.69 | 6.9 | ||
| Estradiol enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | 6.7–7.3 | ||
| Estradiol dienanthate | C3, C17β | Heptanoic acid (×2) | Straight-chain fatty acid | 7 (×2) | 1.82 | 0.55 | 8.1–10.4 | ||
| Estradiol undecylate | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.62 | 0.62 | 9.2–9.8 | ||
| Estradiol stearate | C17β | Octadecanoic acid | Straight-chain fatty acid | 18 | 1.98 | 0.51 | 12.2–12.4 | ||
| Estradiol distearate | C3, C17β | Octadecanoic acid (×2) | Straight-chain fatty acid | 18 (×2) | 2.96 | 0.34 | 20.2 | ||
| Estradiol sulfate | C3 | Sulfuric acid | Water-soluble conjugate | – | 1.29 | 0.77 | 0.3–3.8 | ||
| Estradiol glucuronide | C17β | Glucuronic acid | Water-soluble conjugate | – | 1.65 | 0.61 | 2.1–2.7 | ||
| Estramustine phosphate | C3, C17β | Normustine, phosphoric acid | Water-soluble conjugate | – | 1.91 | 0.52 | 2.9–5.0 | ||
| Polyestradiol phosphate | C3–C17β | Phosphoric acid | Water-soluble conjugate | – | 1.23 | 0.81 | 2.9 | ||
| Footnotes: = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic or cyclic fatty acids. = Relative estradiol content by weight (i.e., relative estrogenic exposure). = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity/hydrophobicity). Retrieved from PubChem, ChemSpider, and DrugBank. = Also known as estradiol normustine phosphate. = Polymer of estradiol phosphate (~13 repeat units). = Relative molecular weight or estradiol content per repeat unit. = log P of repeat unit (i.e., estradiol phosphate). Sources: See individual articles. | |||||||||
See also
References
- ^ Cavalli F, Kaye SB, Hansen HH, Armitage JO, Piccart-Gebhart M (12 September 2009). "Appendix: Endocrine Therapies". Textbook of Medical Oncology (Fourth ed.). CRC Press. pp. 442–. ISBN 978-0-203-09289-7.
- ^ Gunnarsson PO, Norlén BJ (1988). "Clinical pharmacology of polyestradiol phosphate". The Prostate. 13 (4): 299–304. doi:10.1002/pros.2990130405. PMID 3217277. S2CID 33063805.
- Abbou CC, Beaumont V, Chopin D, Deburge JP, Beaumont JL, Auvert J (29 June 2013). "Treatment of prostatic cancer with diethylstilboestrol and detection of the vascular risk". In Smith PH (ed.). Cancer of the Prostate and Kidney. Springer Science & Business Media. pp. 359–. ISBN 978-1-4684-4349-3.
| Salts and covalent derivatives of the estradiol ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This drug article relating to the genito-urinary system is a stub. You can help Misplaced Pages by expanding it. |
This article about a steroid is a stub. You can help Misplaced Pages by expanding it. |