| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Chloro(trifluoro)methane | |||
| Other names
Chlorotrifluoromethane Monochlorotrifluoromethane Trifluorochloromethane Trifluoromethyl chloride Trifluoromonochlorocarbon Arcton 3 Freon 13 Genetron 13 R-13 CFC 13 UN 1022 | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.814 | ||
| EC Number |
| ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
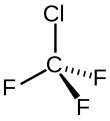
| Chemical formula | CClF3 | ||
| Molar mass | 104.46 g/mol | ||
| Appearance | Colorless gas with sweet odor | ||
| Density | 1.526 g/cm | ||
| Melting point | −181 °C (−293.8 °F; 92.1 K) | ||
| Boiling point | −81.5 °C (−114.7 °F; 191.7 K) | ||
| Solubility in water | 0.009% at 25 °C (77 °F) | ||
| Vapor pressure | 3.263 MPa at 21 °C (70 °F) | ||
| Thermal conductivity | 0.01217 W m K (300 K) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Ozone depletor and asphyxiant | ||
| Flash point | Non-flammable | ||
| Safety data sheet (SDS) | ICSC 0420 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Chlorotrifluoromethane, R-13, CFC-13, or Freon 13, is a non-flammable, non-corrosive, nontoxic chlorofluorocarbon (CFC) and also a mixed halomethane. It is a man-made substance used primarily as a refrigerant. When released into the environment, CFC-13 has a high ozone depletion potential, and long atmospheric lifetime. Only a few other greenhouse gases surpass CFC-13 in global warming potential (GWP). The IPCC AR5 reported that CFC-13's atmospheric lifetime was 640 years.
Production
CFC-13—like all chlorofluorocarbon compounds—contains atoms of carbon (C), chlorine (Cl), and fluorine (F).
It can be prepared by reacting carbon tetrachloride with hydrogen fluoride in the presence of a catalytic amount of antimony pentachloride:
- CCl4 + 3 HF → CClF3 + 3 HCl
This reaction can also produce trichlorofluoromethane (CCl3F), dichlorodifluoromethane (CCl2F2) and tetrafluoromethane (CF4).
Montreal Protocol
Main article: Montreal ProtocolFollowing the unanimous ratification of the 1987 Montreal Protocol—in response to concerns about the role of concentrations of chlorofluorocarbons (CFCs) in ozone layer-depletion in the stratosphere—a process was put into place to gradually phase out and replace CFC-13 and all the other CFCs. Research in the 1980s said that these man-made CFC compound compounds had opened a hole in ozone layer in the upper atmosphere or stratosphere that protects life on earth from UV radiation.
CFC-13's ozone depletion potential (ODP) is high— 1 (CCl3F = 1)—it is categorized as a Class I in the IPCC's list of ozone-depleting substances. CFC-13's radiative efficiency is high which results in a high global warming potential (GWPs) of 13 900 GWP-100 yr that is "surpassed by very few other greenhouse gases." It is categorized as a Class I in the list of ozone-depleting Substances.
Increase in atmospheric abundance of CFC-13 in 2010s
Starting in the 2010s, despite a global ban on the production of CFCs, five of these ozone-damaging emissions were on the rise.
The atmospheric abundance of CFC-13 rose from 3.0 parts per trillion (ppt) in year 2010 to 3.3 ppt in year 2020 based on analysis of air samples gathered from sites around the world. Contrary to the Montreal Protocol, the atmospheric emissions of CFC-13 and four other chlorofluorocarbons (CFCs), increased between 2010 and 2020.
As of 2023, the drivers behind the increase in CFC-13 and CFC-112a emissions were not certain.
Physical properties
The IPCC AR5 reported that CFC-13's Atmospheric lifetime was 640 years.
| Property | Value |
|---|---|
| Density (ρ) at -127.8 °C (liquid) | 1.603 g⋅cm |
| Density (ρ) at boiling point (gas) | 6.94 kg⋅m |
| Density (ρ) at 15 °C (gas) | 4.41 g⋅cm |
| Triple point temperature (Tt) | |
| Critical temperature (Tc) | 28.8 °C (302 K) |
| Critical pressure (pc) | 3.86 MPa (38.6 bar) |
| Critical density (ρc) | 5.5 mol⋅L |
| Latent heat of vaporization at boiling point | 149.85 kJ⋅kg |
| Specific heat capacity at constant pressure (Cp) at -34.4 °C | 0.06 kJ⋅mol⋅K |
| Specific heat capacity at constant volume (CV) at -34.4 °C | 0.051 kJ⋅mol⋅K |
| Heat capacity ratio (к) at -34.4 °C | 1.168016 |
| Compressibility Factor (Z) at 15 °C | 0.9896 |
| Acentric factor (ω) | 0.17166 |
| Viscosity (η) at 0 °C (gas) | 13.3 mPa⋅s (0.0133 cP) |
| Viscosity (η) at 25 °C (gas) | 14.1 mPa⋅s (0.01440 cP) |
| Ozone depletion potential (ODP) | 1(CCl3F = 1) |
| Global warming potential (GWP) | 14,000 (CO2 = 1) |
| Atmospheric lifetime | 640 years |
See also
References
- Touloukian, Y.S., Liley, P.E., and Saxena, S.C. Thermophysical properties of matter - the TPRC data series. Volume 3. Thermal conductivity - nonmetallic liquids and gases. Data book. 1970.
- Siegemund, Günter; Schwertfeger, Werner; Feiring, Andrew; Smart, Bruce; Behr, Fred; Vogel, Herward; McKusick, Blaine (2002). "Fluorine Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_349. ISBN 978-3527306732.
- ^ Vollmer, Martin; Young, Dickon; Trudinger, Cathy; Mühle, Jens; Henne, Stephan; Rigby, Matt; Park, Sunyoung; Li, Shihong; Guillevic, Myriam; Mitrevski, Blagoj; Harth, Christina; Miller, Benjamin; Reimann, Stefan; Yao, Bo; Steele, L.; Wyss, Simon; Lunder, Chris; Arduini, Jgor; McCulloch, Archie; Simmonds, Peter (October 10, 2017). "Atmospheric histories and emissions of chlorofluorocarbons CFC-13 (CClF3), CFC-114 (C2Cl2F4), and CFC-115 (C2ClF5)". Atmospheric Chemistry and Physics Discussions. 2017 (39). doi:10.5194/acp-2017-935. hdl:1721.1/116270.
- ^ "Chapter 8". AR5 Climate Change 2013: The Physical Science Basis. p. 731.
- ^ Ashworth, James (April 3, 2023). "Mystery emissions of ozone-damaging gases are fuelling climate change". Natural History Museum. Retrieved April 3, 2023.
- Elkins, James.W. (2013). "Halocarbons and other Atmospheric Trace Species". NOAA Global Monitoring Laboratory (Press release). Retrieved April 3, 2023 – via US Department of Commerce and NOAA.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 304. ISBN 978-0-08-037941-8.
- Allen, Kate (April 3, 2023). "Remember ozone-destroying CFCs? They're on the rise again. And the source is a mystery". The Star. Retrieved April 3, 2023.
- ^ "Class I Ozone-depleting Substances". Science - Ozone Layer Protection. US EPA. 2007. Archived from the original on 2010-12-10. Retrieved 2010-12-16.
- "AGAGE Data and Figures". Massachusetts Institute of Technology. Retrieved 2021-02-11.
- ^ Western, Luke M.; Vollmer, Martin K.; Krummel, Paul B.; Adcock, Karina E.; Fraser, Paul J.; Harth, Christina M.; Langenfelds, Ray L.; Montzka, Stephen A.; Mühle, Jens; O’Doherty, Simon; Oram, David E.; Reimann, Stefan; Rigby, Matt; Vimont, Isaac; Weiss, Ray F.; Young, Dickon; Laube, Johannes C. (April 3, 2023). "Global increase of ozone-depleting chlorofluorocarbons from 2010 to 2020". Nature Geoscience. 16 (4): 309–313. Bibcode:2023NatGe..16..309W. doi:10.1038/s41561-023-01147-w. hdl:1983/9e103fef-e61c-49c7-a1a3-902540ec1d7c. ISSN 1752-0908. S2CID 257941769. Retrieved April 3, 2023.
- Forster, Piers; Ramaswamy, Venkatachalam; Artaxo, Paulo; Berntsen, Terje; Betts, Richard; Fahey, David W; Haywood, James; Lean, Judith; Lowe, David C; Raga, Graciela; Schulz, Michael; Dorland, Robert Van; Bodeker, G; Etheridge, D; Foukal, P; Fraser, P; Geller, M; Joos, F; Keeling, C D; Keeling, R; Kinne, S; Lassey, K; Oram, D; O’Shaughnessy, K; Ramankutty, N; Reid, G; Rind, D; Rosenlof, K; Sausen, R; Schwarzkopf, D; Solanki, S K; Stenchikov, G; Stuber, N; Takemura, T; Textor, C; Wang, R; Weiss, R; Whorf, T; Nakajima, Teruyuki; Ramanathan, Veerabhadran; Ramaswamy, V; Artaxo, P; Berntsen, T; Betts, R; Fahey, D W; Haywood, J; Lean, J; Lowe, D C; Myhre, G; Nganga, J; Prinn, R; Raga, G; Schulz, M; Dorland, R Van. "Changes in Atmospheric Constituents and in Radiative Forcing". International Panel of Climate Change (IPCC). AR4 Climate Change 2007: The Physical Science Basis.
External links
- MSDS at mathesontrigas.com
- International Chemical Safety Card 0420
- Entry at Air Liquide gas encyclopaedia Archived 2011-07-07 at the Wayback Machine
- The crystal structure of chlorotrifluoromethane, CF3Cl; neutron powder diffraction and constrained refinement
- Termochemical data table
| Halomethanes | |
|---|---|
| Unsubstituted | |
| Monosubstituted | |
| Disubstituted | |
| Trisubstituted | |
| Tetrasubstituted | |
| * Chiral compound. | |