| |
| Names | |
|---|---|
| IUPAC name Lutetium(III) chloride | |
| Other names Lutetium chloride, lutetium trichloride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.030.205 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | LuCl3 |
| Molar mass | 281.325 g/mol |
| Appearance | colorless or white monoclinic crystals |
| Density | 3.98 g/cm |
| Melting point | 925 °C (1,697 °F; 1,198 K) |
| Boiling point | sublimes above 750°C |
| Solubility in water | soluble |
| Structure | |
| Crystal structure | Monoclinic, mS16 |
| Space group | C2/m, No. 12 |
| Pharmacology | |
| License data | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Irritant |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Related compounds | |
| Other anions | Lutetium(III) oxide |
| Other cations | Ytterbium(III) chloride Scandium(III) chloride Yttrium(III) chloride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
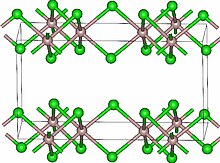
Lutetium(III) chloride or lutetium trichloride is the chemical compound composed of lutetium and chlorine with the formula LuCl3. It forms hygroscopic white monoclinic crystals and also a hygroscopic hexahydrate LuCl3·6H2O. Anhydrous lutetium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral lutetium ions.
Lutetium-177, a radioisotope that can be derived from lutetium(III) chloride, is used in targeted cancer therapies. When lutetium-177 is attached to molecules that specifically target cancer cells, it can deliver localized radiation to destroy those cells while sparing surrounding healthy tissue. This makes lutetium-177-based treatments especially valuable for cancers that are difficult to treat with traditional methods, such as neuroendocrine tumors and prostate cancer. Additionally, lutetium(III) chloride is used in scintillators, materials that emit light when exposed to radiation. These scintillators are crucial in detectors for gamma rays and other high-energy particles, used in both medical diagnostics and in scientific research.
Reactions
Pure lutetium metal can be produced from lutetium(III) chloride by heating it together with elemental calcium:
See also
References
- "Chemistry: Periodic Table: Lutetium: compound data (lutetium (III) chloride)". WebElements. Retrieved 2024-09-06.
- Perry, Dale L.; Phillips, Sidney L. (1995), Handbook of Inorganic Compounds, CRC Press, p. 232, ISBN 0-8493-8671-3, retrieved 2008-06-27
- ^ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, p. 472, ISBN 0-8493-0594-2, retrieved 2008-06-27
- "450960 Lutetium(III) chloride anhydrous, powder, 99.99% trace metals basis". Sigma-Aldrich. Retrieved 2008-06-27.
- "Lutetium chloride". pubchem.ncbi.nlm.nih.gov.
- "Lutetium(III) chloride hexahydrate 542075". Sigma-Aldrich. Retrieved 2019-07-24.
- Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- Sgouros, George; Bodei, Lisa; McDevitt, Michael R.; Nedrow, Jessie R. (September 2020). "Radiopharmaceutical therapy in cancer: clinical advances and challenges". Nature Reviews Drug Discovery. 19 (9): 589–608. doi:10.1038/s41573-020-0073-9. ISSN 1474-1784. PMC 7390460.
- Vyas, Madhusudan (2021-05-01). "Lutetium-177: a flexible radionuclide therapeutic options". Journal of Nuclear Medicine. 62 (supplement 1): 3039–3039. ISSN 0161-5505.
- Dash, Ashutosh; Pillai, Maroor Raghavan Ambikalmajan; Knapp, Furn F. (2015-06-01). "Production of 177Lu for Targeted Radionuclide Therapy: Available Options". Nuclear Medicine and Molecular Imaging. 49 (2): 85–107. doi:10.1007/s13139-014-0315-z. ISSN 1869-3482. PMC 4463871. PMID 26085854.
- Vogel, W. V.; van der Marck, S. C.; Versleijen, M. W. J. (2021-07-01). "Challenges and future options for the production of lutetium-177". European Journal of Nuclear Medicine and Molecular Imaging. 48 (8): 2329–2335. doi:10.1007/s00259-021-05392-2. ISSN 1619-7089. PMC 8241800. PMID 33974091.
- Das, Tapas; Banerjee, Sharmila (2016). "Theranostic Applications of Lutetium-177 in Radionuclide Therapy". Current Radiopharmaceuticals. 9 (1): 94–101. doi:10.2174/1874471008666150313114644. ISSN 1874-4729. PMID 25771364.
- Patnaik, Pradyot (2004), Handbook of Inorganic Chemicals, Amsterdam: McGraw-Hill Professional, p. 244, ISBN 0-07-049439-8, retrieved 2008-06-27
| Lutetium compounds | |
|---|---|
| Lanthanide salts of halides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||