| |
| Names | |
|---|---|
| Other names Manganese(II) stearate, manganese distearate, manganese(2+) dioctadecanoate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.020.110 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C 36H 70MnO 4 |
| Molar mass | 621.89 |
| Appearance | Pale pink powder |
| Density | g/cm |
| Boiling point | 359.4 °C (678.9 °F; 632.5 K) |
| Solubility in water | insoluble |
| Hazards | |
| GHS labelling: | |
| Signal word | Warning |
| Hazard statements | H302, H312, H315, H319, H332, H335 |
| Flash point | 162.4 °C (324.3 °F; 435.5 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
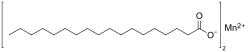
Manganese stearate is a metal-organic compound, a salt of manganese and stearic acid with the chemical formula C
36H
70MnO
4. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Synthesis
Manganese stearate is synthesized by the reaction of stearic acid with sodium hydroxide, followed by reacting with manganese chloride.
Also, the reaction of manganese(II) acetate with stearic acid.
Physical properties
The compound forms pale pink powder.
Insoluble in water.
Uses
The compound is used in organic synthesis reactions.
Also as an oxidant additive for oxo-biodegradable polymers (for example, high-density polyethylene).
References
- "Manganese Stearate". American Elements. Retrieved 6 March 2023.
- "NCATS Inxight Drugs — MANGANESE STEARATE". National Center for Advancing Translational Sciences. Retrieved 6 March 2023.
- "CAS 3353-05-7 Manganese Stearate - Alfa Chemistry". alfa-chemistry.com. Retrieved 6 March 2023.
- Aras, Neny Rasnyanti M.; Arcana, I Made (2015). "Synthesis of manganese stearate for high density polyethylene (HDPE) and its biodegradation". AIP Conference Proceedings. 1677 (1): 070024. Bibcode:2015AIPC.1677g0024A. doi:10.1063/1.4930728. Retrieved 6 March 2023.
- "manganese stearate". chemsrc.com. Retrieved 6 March 2023.
- ^ "Manganese Stearate | CAS 3353-05-7". Santa Cruz Biotechnology. Retrieved 6 March 2023.
- Roy, Prasun Kumar; Singh, Priyanka; Kumar, Devendra; Rajagopal, Chitra (2010). "Manganese stearate initiated photo-oxidative and thermo-oxidative degradation of LDPE, LLDPE and their blends". Journal of Applied Polymer Science. 117: 524–533. doi:10.1002/app.31252.
| Manganese compounds | |
|---|---|
| Manganese(−I) | |
| Manganese(0) | |
| Manganese(I) | |
| Manganese(II) | |
| Manganese(II,III) | |
| Manganese(II,IV) | |
| Manganese(III) | |
| Manganese(IV) | |
| Manganese(V) | |
| Manganese(VI) | |
| Manganese(VII) | |