| |

| |
| Names | |
|---|---|
| Other names Hydrogen pentacarbonylmanganate(−I) (7CI); Manganese, pentacarbonylhydro- (8CI); Hydridomanganese pentacarbonyl; Hydridopentacarbonylmanganese; Manganese pentacarbonyl hydride; Pentacarbonylhydromanganese; Pentacarbonylmanganese hydride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
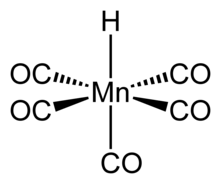
| Chemical formula | HMn(CO)5 |
| Molar mass | 195.99799 g/mol |
| Appearance | At room temperature, it is liquid and colorless. Below its melting point, it may be sublimed in vacuum. |
| Acidity (pKa) | 7.1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Pentacarbonylhydridomanganese is an organometallic compound with formula HMn(CO)5. This compound is one of the most stable "first-row" transition metal hydrides.
Preparation
It was first reported in 1931. Of the several ways to produce this compound, is the protonation of the pentacarbonyl manganate anion. The latter is formed from reduction of dimanganese decacarbonyl, e.g., with superhydride:
- 2 LiHB(C2H5)3 + Mn2(CO)10 → 2 LiMn(CO)5 + H2 + 2 B(C2H5)3
- Li[Mn(CO)5] + CF3SO3H → HMn(CO)5 + CF3SO3Li
Salts of
can be isolated as crystalline PPN
(μ-nitrido—bis-(triphenylphosphorus)) salt, which is smoothly protonated by CF
3SO
3H.
- PPN + CF
3SO
3H → HMn(CO)5 + PPN
CF
3SO
3
This compound can also be formed by the hydrolysis of pentacarbonyl(trimethylsilyl)manganese:
- (CO)5MnSiMe3 + H2O → HMn(CO)5 + Me3SiOH (Me = CH3)
Structure and properties
The structure of HMn(CO)5 has been studied by many methods including X-ray diffraction, neutron diffraction, and electron diffraction. HMn(CO)5 can be related to the structure of a hexacarbonyl complex such as Mn(CO)
6, and therefore has similar properties. The compound has octahedral symmetry and its molecular point group is C4v. The H-Mn bond length is 1.44 ± 0.03 Å. Gas phase electron diffraction analysis confirms these conclusions.
Main reactions
The pKa of HMn(CO)5 in water is 7.1. It is thus comparable to hydrogen sulfide, a common inorganic acid, in its acidity.
A common reaction involving HMn(CO)5 is substitution of the CO ligands by organophosphines, as occurs both thermally and photochemically. In this way the many derivatives form of the type HMn(CO)5-x(PR3)x. (R here need not be a purely hydrocarbon component; it may, for instance, be OEt, where Et = ethyl group.)
HMn(CO)5 can be used to reduce olefins and other organic compounds, as well as metal halides.
It can be methylated with diazomethane.
- HMn(CO)5 + CH2N2 → Mn(CO)5CH3 + N2
Notes
References
- ^ Eley, D.D.; Pines, Herman; Weisz, P.B. Advances In Catalysis. 32. 385. ISBN 978-0-12-007832-5
- Hieber, W. Leutert, F. Naturwissenschaften. 1931. 360.
- ^ Hunter, Alan D; Bianconi, Larry J; DiMuzio, Steven J; Braho, Dianne L. Synthesis and Structure- Property Relationships in η6-Arene) Cr(CO)3 Chemistry: From Guided Experiments to Discovery Research. J. Chem. Educ. 75. 1998. 891. doi:10.1021/ed075p891
- Finn, M.G. Pentacarbonyl(trimethylsilyl)manganese. Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rp022s
- ^ Kukolich, S.G. Microwave Spectrum and Molecular Structure for Manganese Pentacarbonyl Hydride. 33. 1994. 1217-1219
- Fenske, Richard. Electronic Structure and Bonding in Manganese Pentacarbonyl Halides and Hydride. Inorganic Chemistry. 9. 1970. 1053-1060.
- Liu, Xian-mei; Wang, Chao-yang; Qian-shu; Xie; Yaoming; King, R. Bruce; Schaefer, Henry F., III. Mononuclear and binuclear manganese carbonyl hydrides. Dalton Trans., 2009, 3774-3785, doi:10.1039/b822913a
- Morris, Robert H. (2016-08-10). "Brønsted–Lowry Acid Strength of Metal Hydride and Dihydrogen Complexes". Chemical Reviews. 116 (15): 8588–8654. doi:10.1021/acs.chemrev.5b00695. hdl:1807/78047. ISSN 0009-2665. PMID 26963836.
- Albertin, Gabriele. Cationic Molecular Hydrogen Complexes of Mn (I). Organometallics. 16. 1997. 4959-4969.
| Manganese compounds | |
|---|---|
| Manganese(−I) | |
| Manganese(0) | |
| Manganese(I) | |
| Manganese(II) | |
| Manganese(II,III) | |
| Manganese(II,IV) | |
| Manganese(III) | |
| Manganese(IV) | |
| Manganese(V) | |
| Manganese(VI) | |
| Manganese(VII) | |