| |
| Names | |
|---|---|
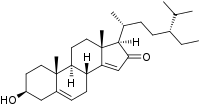
| IUPAC name 3β-Hydroxystigmasta-5,14-dien-16-one | |
| Systematic IUPAC name (1R,3bR,7S,9aR,9bS,11aR)-1--7-hydroxy-9a,11a-dimethyl-1,3b,4,6,7,8,9,9a,9b,10,11,11a-dodecahydro-2H-cyclopentaphenanthren-2-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C29H46O2 |
| Molar mass | 426.685 g·mol |
| Melting point | 160 °C (320 °F; 433 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Momordenol (3β-hydroxy-stigmasta-5,14-dien-16-one) is a natural chemical compound, a sterol found in the fresh fruit of the bitter melon (Momordica charantia).
The compound is soluble in ethyl acetate and methanol but not in pure chloroform or petrol. It crystallizes as fine needles that melt at 160–161 °C. It was isolated in 1997 by S. Begum and others.
See also
References
- ^ Begum, Sabira; Ahmed, Mansoor; Siddiqui, Bina S.; Khan, Abdullah; Saify, Zafar S.; Arif, Mohammed (1997). "Triterpenes, A sterol and a monocyclic alcohol from Momordica charantia". Phytochemistry. 44 (7): 1313–1320. Bibcode:1997PChem..44.1313B. doi:10.1016/S0031-9422(96)00615-2.
| Phytosterols | |
|---|---|
| C28 |
|
| C29 | |