| |
| Names | |
|---|---|
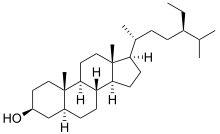
| IUPAC name 5α-Stigmastan-3β-ol | |
| Systematic IUPAC name (1R,3aS,3bR,5aS,7S,9aS,9bS,11aR)-1--9a,11a-dimethylhexadecahydro-1H-cyclopentaphenanthren-7-ol | |
| Other names (3β)-Stigmastan-3-ol; (3β,5α)-Stigmastan-3-ol; β-Sitostanol; Dihydrositosterin; Dihydrositosterol; Dihydro-β-sitosterol; Fucostanol; Spinastanol; 24α-Ethylcholestanol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.345 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C29H52O |
| Molar mass | 416.734 g·mol |
| Boiling point | 139.4 to 139.8 °C (282.9 to 283.6 °F; 412.5 to 412.9 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Stigmastanol (sitostanol) is a phytosterol found in a variety of plant sources. Similar to sterol esters and stanol esters, stigmastanol inhibits the absorption of cholesterol from the diet. Animal studies suggest that it also inhibits biosynthesis of cholesterol in the liver.
Stigmastanol is the product of the reduction of β-sitosterol and the hydrogenation of stigmasterol.
See also
- Stigmasterol, a closely related sterol
References
- Sandqvist, Hakan; Bengtsson, Edvard (1931). "The empirical formula of sitosterol". Berichte der Deutschen Chemischen Gesellschaft B. 64: 2167–2171. doi:10.1002/cber.19310640849.
- ^ Batta, Ashok K.; Xu, Guorong; Honda, Akira; Miyazaki, Teruo; Salen, Gerald (2006). "Stigmasterol reduces plasma cholesterol levels and inhibits hepatic synthesis and intestinal absorption in the rat". Metabolism: Clinical and Experimental. 55 (3): 292–299. doi:10.1016/j.metabol.2005.08.024. PMID 16483871.
- Heinemann T, Pietruck B, Kullak-Ublick G, von Bergmann K (1988). "Comparison of sitosterol and sitostanol on inhibition of intestinal cholesterol absorption". Agents and Actions. Supplements. 26: 117–122. PMID 3265272.
- Heinemann T, Kullak-Ublick GA, Pietruck B, von Bergmann K (1991). "Mechanisms of action of plant sterols on inhibition of cholesterol absorption. Comparison of sitosterol and sitostanol". European Journal of Clinical Pharmacology. 40 (Suppl 1): S59–63. doi:10.1007/BF01409411. PMID 2044646. S2CID 23279253.
- Thomasson Crompton, David William; Nickol, Brent B. (1985). Biology of the Acanthocephala. Cambridge University Press. p. 185. ISBN 9780521246743.
- Paxena, P. B. (2007). Chemistry of Alkaloids. Discovery Publishing Hous. p. 231. ISBN 9788183563161.
| Phytosterols | |
|---|---|
| C28 |
|
| C29 | |
This article about a steroid is a stub. You can help Misplaced Pages by expanding it. |