
Neurosteroids, also known as neuroactive steroids, are endogenous or exogenous steroids that rapidly alter neuronal excitability through interaction with ligand-gated ion channels and other cell surface receptors. The term neurosteroid was coined by the French physiologist Étienne-Émile Baulieu and refers to steroids synthesized in the brain. The term, neuroactive steroid refers to steroids that can be synthesized in the brain, or are synthesized by an endocrine gland, that then reach the brain through the bloodstream and have effects on brain function. The term neuroactive steroids was first coined in 1992 by Steven Paul and Robert Purdy. In addition to their actions on neuronal membrane receptors, some of these steroids may also exert effects on gene expression via nuclear steroid hormone receptors. Neurosteroids have a wide range of potential clinical applications from sedation to treatment of epilepsy and traumatic brain injury. Ganaxolone, a synthetic analog of the endogenous neurosteroid allopregnanolone, is under investigation for the treatment of epilepsy.
Classification
See also: List of neurosteroidsBased on differences in activity and structure, neurosteroids can be broadly categorized into several different major groupings.
Inhibitory neurosteroids
These neurosteroids exert inhibitory actions on neurotransmission. They act as positive allosteric modulators of the GABAA receptor (especially δ subunit-containing isoforms), and possess, in no particular order, antidepressant, anxiolytic, stress-reducing, rewarding, prosocial, antiaggressive, prosexual, sedative, pro-sleep, cognitive and memory-impairing, analgesic, anesthetic, anticonvulsant, neuroprotective, and neurogenic effects.
Major examples include tetrahydrodeoxycorticosterone (THDOC), the androstane 3α-androstanediol, the cholestane cholesterol and the pregnanes pregnanolone (eltanolone), allopregnanolone (3α,5α-THP).
Excitatory neurosteroids
These neurosteroids have excitatory effects on neurotransmission. They act as potent negative allosteric modulators of the GABAA receptor, weak positive allosteric modulators of the NMDA receptor, and/or agonists of the σ1 receptor, and mostly have antidepressant, anxiogenic, cognitive and memory-enhancing, convulsant, neuroprotective, and neurogenic effects.
Major examples include the pregnanes pregnenolone sulfate (PS), epipregnanolone, and isopregnanolone (sepranolone), the androstanes dehydroepiandrosterone (DHEA; prasterone), and dehydroepiandrosterone sulfate (DHEA-S; prasterone sulfate), and the cholestane 24(S)-hydroxycholesterol (NMDA receptor-selective; very potent).
Pheromones
Main article: Pheromone § Axillary steroidsPheromones are neurosteroids that influence brain activity, notably hypothalamic function, via activation of vomeronasal receptor cells.
Possible human pheromones include the androstanes androstadienol, androstadienone, androstenol, and androstenone and the estrane estratetraenol.
Other neurosteroids
Certain other endogenous steroids, such as pregnenolone, progesterone, estradiol, and corticosterone are also neurosteroids. However, unlike those listed above, these neurosteroids do not modulate the GABAA or NMDA receptors, and instead affect various other cell surface receptors and non-genomic targets. Also, many endogenous steroids, including pregnenolone, progesterone, corticosterone, deoxycorticosterone, DHEA, and testosterone, are metabolized into (other) neurosteroids, effectively functioning as so-called proneurosteroids.
Biosynthesis
Neurosteroids are synthesized from cholesterol, which is converted into pregnenolone and then into all other endogenous steroids. Neurosteroids are produced in the brain after local synthesis or by conversion of peripherally-derived adrenal steroids or gonadal steroids. They accumulate especially in myelinating glial cells, from cholesterol or steroidal precursors imported from peripheral sources. 5α-reductase type I and 3α-hydroxysteroid dehydrogenase are involved in the biosynthesis of inhibitory neurosteroids, while 3β-hydroxysteroid dehydrogenase and hydroxysteroid sulfotransferases are involved in excitatory neurosteroid production.
Function
Some major known biological functions of neurosteroids include modulation of neural plasticity, learning and memory processes, behavior, and seizure susceptibility, as well as responses to stress, anxiety, and depression. Neurosteroids also appear to play an important role in various sexually-dimorphic behaviors and emotional responses.
Acute stress elevates the levels of inhibitory neurosteroids like allopregnanolone, and these neurosteroids are known to counteract many of the effects of stress. This is similar to the case of endorphins, which are released in response to stress and physical pain and counteract the negative subjective effects of such states. As such, it has been suggested that one of the biological functions of these neuromodulators may be to help maintain emotional homeostasis. Chronic stress has been associated with diminished levels of allopregnanolone and altered allopregnanolone stress responsivity, psychiatric disorders, and hypothalamic-pituitary-adrenal axis dysregulation.
It is thought that fluctuations in the levels of inhibitory neurosteroids during the menstrual cycle and pregnancy play an important role in a variety of women's conditions, including premenstrual syndrome (PMS), premenstrual dysphoric disorder (PMDD), postpartum depression (PPD), postpartum psychosis, and catamenial epilepsy. In addition, it is thought that changes in neurosteroid levels may be involved in the changes in mood, anxiety, and sexual desire that occur during puberty in both sexes and during menopause in women.
Elevated levels of inhibitory neurosteroids, namely allopregnanolone, can produce paradoxical effects, such as negative mood, anxiety, irritability, and aggression. This appears to be because these neurosteroids, like other positive allosteric modulators of the GABAA receptor such as the benzodiazepines, barbiturates, and ethanol, possess biphasic, U-shaped actions – moderate levels (in the range of 1.5–2 nM/L total alloprogesterone, which are approximately equivalent to luteal phase levels) inhibit the activity of the GABAA receptor, while lower and higher concentrations facilitate the activity of the receptor.
Biological activity
Sigma-1 receptor
| Compound | Ki (nM) | Action | Species | Ref |
|---|---|---|---|---|
| Progesterone | 268 | Antagonist | Guinea pig | |
| Deoxycorticosterone | 938 | Unknown | Guinea pig | |
| Testosterone | 1,014 | Unknown | Guinea pig | |
| Pregnenolone | ND | Agonist | ND | ND |
| Pregnenolone sulfate | 3,198 | Agonist | Guinea pig | |
| DHEATooltip Dehydroepiandrosterone | 3,700 | Agonist | ? | |
| DHEA-STooltip Dehydroepiandrosterone sulfate | ND | Agonist | ND | ND |
| Corticosterone | 4,074 | Unknown | Guinea pig |
Therapeutic applications
Anesthesia
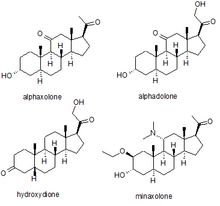
Several synthetic neurosteroids have been used as sedatives for the purpose of general anaesthesia for carrying out surgical procedures. The best known of these are alphaxolone, alphadolone, hydroxydione, and minaxolone. The first of these to be introduced was hydroxydione, which is the esterified 21-hydroxy derivative of 5β-pregnanedione. Hydroxydione proved to be a useful anaesthetic drug with a good safety profile, but was painful and irritating when injected probably due to poor water solubility. This led to the development of newer neuroactive steroids. The next drug from this family to be marketed was a mixture of alphaxolone and alphadolone, known as Althesin. This was withdrawn from human use due to rare but serious toxic reactions, but is still used in veterinary medicine. The next neurosteroid anaesthetic introduced into human medicine was the newer drug minaxolone, which is around three times more potent than althesin and retains the favourable safety profile, without the toxicity problems seen with althesin. However this drug was also ultimately withdrawn, not because of problems in clinical use, but because animal studies suggested potential carcinogenicity and since alternative agents were available it was felt that the possible risk outweighed the benefit of keeping the drug on the market.
Ganaxolone

The neurosteroid ganaxolone, an analog of the progesterone metabolite allopregnanolone, has been extensively investigated in animal models and is currently in clinical trials for the treatment of epilepsy. Neurosteroids, including ganaxolone have a broad spectrum of activity in animal models. They may have advantages over other GABAA receptor modulators, notably benzodiazepines, in that tolerance does not appear to occur with extended use.
A randomized, placebo controlled, 10-week phase 2 clinical trial of orally administered ganaxolone in adults with partial onset seizure demonstrated that the treatment is safe, well tolerated and efficacious. The drug continued to demonstrate efficacy in a 104-week open label extension. Data from non-clinical studies suggest that ganaxolone may have low risk for use in pregnancy. In addition to use in the treatment of epilepsy, the drug has potential in the treatment of a broad range of neurological and psychiatric conditions. Proof-of-concept studies are currently underway in posttraumatic stress disorder and fragile X syndrome. Ganaxolone was approved for medical use in the United States in March 2022.
Catamenial epilepsy
Researchers have suggested the use of so-called "neurosteroid replacement therapy" as a way of treating catamenial epilepsy with neuroactive steroids such as ganaxolone during the period of the menstrual cycle when seizure frequency increases. Micronized progesterone, which behaves reliably as a prodrug to allopregnanolone, has been suggested as a treatment for catamenial epilepsy in the same manner.
Allopregnanolone
Allopregnanolone (SAGE-547) is under development as an intravenous therapy for the treatment of super-refractory status epilepticus, postpartum depression, and essential tremor.
Other applications
4,16-Androstadien-3β-ol (PH94B, Aloradine) is a synthetic pheromone, or pherine, neurosteroid which is under investigation for the treatment of anxiety disorders in women.
3β-Methoxypregnenolone (MAP-4343), or pregnenolone 3β-methyl ether, is a synthetic neuroactive steroid and pregnenolone derivative that interacts with microtubule-associated protein 2 (MAP2) in a similar manner to pregnenolone and is under development for potential clinical use for indications such as the treatment of brain and spinal cord injury and depressive disorders.
Role in antidepressant action
Certain antidepressant drugs such as fluoxetine and fluvoxamine, which are generally thought to affect depression by acting as selective serotonin reuptake inhibitors (SSRIs), have also been found to normalize the levels of certain neurosteroids (which are frequently deficient in depressed patients) at doses that are inactive in affecting the reuptake of serotonin. This suggests that other actions involving neurosteroids may also be at play in the effectiveness of these drugs against depression.
The 3α-hydroxysteroid dehydrogenase (3α-HSD) enzyme is induced by certain (SSRIs), including fluoxetine, fluvoxamine, sertraline, and paroxetine, as well as by certain other antidepressants like venlafaxine and mirtazapine, and these antidepressants have been found to increase inhibitory neurosteroid levels. Enhancement of biosynthesis of inhibitory neurosteroids has been implicated in the antidepressant and anxiolytic effects of some of the SSRIs.
Benzodiazepine effects on neurosteroids
Benzodiazepines may influence neurosteroid metabolism by virtue of their actions on translocator protein (TSPO; "peripheral benzodiazepine receptor"). The pharmacological actions of benzodiazepines at the GABAA receptor are similar to those of neurosteroids, although the localization of benzodiazepine and neurosteroid-sensitive GABAA receptor subtypes vary. Factors which affect the ability of individual benzodiazepines to alter neurosteroid levels may depend upon whether the individual benzodiazepine drug interacts with TSPO. Some benzodiazepines may also inhibit neurosteroidogenic enzymes reducing neurosteroid synthesis.
See also
References
- Paul SM, Purdy RH (March 1992). "Neuroactive steroids". FASEB Journal. 6 (6): 2311–22. doi:10.1096/fasebj.6.6.1347506. PMID 1347506. S2CID 221753076.
- Lan NC, Gee KW (December 1994). "Neuroactive steroid actions at the GABAA receptor". Hormones and Behavior. 28 (4): 537–44. doi:10.1006/hbeh.1994.1052. PMID 7729823. S2CID 40697424.
- ^ Reddy DS (2010). "Neurosteroids". Sex Differences in the Human Brain, their Underpinnings and Implications. Progress in Brain Research. Vol. 186. pp. 113–37. doi:10.1016/B978-0-444-53630-3.00008-7. ISBN 9780444536303. PMC 3139029. PMID 21094889.
- Reddy DS, Rogawski MA (2012). "Neurosteroids — Endogenous Regulators of Seizure Susceptibility and Role in the Treatment of Epilepsy". In Noebels JL, Avoli M, Rogawski MA, et al. (eds.). Jasper's Basic Mechanisms of the Epilepsies . 4th edition. Bethesda (MD): National Center for Biotechnology Information (US). National Center for Biotechnology Information (US). PMID 22787590.
- ^ Srivastava DP, Waters EM, Mermelstein PG, Kramár EA, Shors TJ, Liu F (November 2011). "Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry". The Journal of Neuroscience. 31 (45): 16056–63. doi:10.1523/JNEUROSCI.4097-11.2011. PMC 3245715. PMID 22072656.
- ^ Reddy DS, Rogawski MA (April 2009). "Neurosteroid replacement therapy for catamenial epilepsy". Neurotherapeutics. 6 (2): 392–401. doi:10.1016/j.nurt.2009.01.006. PMC 2682439. PMID 19332335.
- Morrow AL (October 2007). "Recent developments in the significance and therapeutic relevance of neuroactive steroids--Introduction to the special issue". Pharmacology & Therapeutics. 116 (1): 1–6. doi:10.1016/j.pharmthera.2007.04.003. PMC 2047816. PMID 17531324.
- Dubrovsky BO (February 2005). "Steroids, neuroactive steroids and neurosteroids in psychopathology". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 29 (2): 169–92. doi:10.1016/j.pnpbp.2004.11.001. PMID 15694225. S2CID 36197603.
- ^ Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS (January 2013). "Progress report on new antiepileptic drugs: a summary of the Eleventh Eilat Conference (EILAT XI)". Epilepsy Research. 103 (1): 2–30. doi:10.1016/j.eplepsyres.2012.10.001. PMID 23219031.
- Rougé-Pont F, Mayo W, Marinelli M, Gingras M, Le Moal M, Piazza PV (July 2002). "The neurosteroid allopregnanolone increases dopamine release and dopaminergic response to morphine in the rat nucleus accumbens". The European Journal of Neuroscience. 16 (1): 169–73. doi:10.1046/j.1460-9568.2002.02084.x. PMID 12153544. S2CID 9953445.
- ^ Frye CA (December 2009). "Neurosteroids' effects and mechanisms for social, cognitive, emotional, and physical functions". Psychoneuroendocrinology. 34 (Suppl 1): S143-61. doi:10.1016/j.psyneuen.2009.07.005. PMC 2898141. PMID 19656632.
- Pinna G, Costa E, Guidotti A (February 2005). "Changes in brain testosterone and allopregnanolone biosynthesis elicit aggressive behavior". Proceedings of the National Academy of Sciences of the United States of America. 102 (6): 2135–40. Bibcode:2005PNAS..102.2135P. doi:10.1073/pnas.0409643102. PMC 548579. PMID 15677716.
- Terán-Pérez G, Arana-Lechuga Y, Esqueda-León E, Santana-Miranda R, Rojas-Zamorano JÁ, Velázquez Moctezuma J (October 2012). "Steroid hormones and sleep regulation". Mini Reviews in Medicinal Chemistry. 12 (11): 1040–8. doi:10.2174/138955712802762167. PMID 23092405.
- Patte-Mensah C, Meyer L, Taleb O, Mensah-Nyagan AG (February 2014). "Potential role of allopregnanolone for a safe and effective therapy of neuropathic pain". Progress in Neurobiology. 113: 70–8. doi:10.1016/j.pneurobio.2013.07.004. PMID 23948490. S2CID 207407077.
- Hénin J, Salari R, Murlidaran S, Brannigan G (May 2014). "A predicted binding site for cholesterol on the GABAA receptor". Biophysical Journal. 106 (9): 1938–1949. Bibcode:2014BpJ...106.1938H. doi:10.1016/j.bpj.2014.03.024. PMC 4017285. PMID 24806926.
- Levitan I, Singh DK, Rosenhouse-Dantsker A (2014). "Cholesterol binding to ion channels". Frontiers in Physiology. 5: 65. doi:10.3389/fphys.2014.00065. PMC 3935357. PMID 24616704.
- Paul SM, Doherty JJ, Robichaud AJ, Belfort GM, Chow BY, Hammond RS, et al. (October 2013). "The major brain cholesterol metabolite 24(S)-hydroxycholesterol is a potent allosteric modulator of N-methyl-D-aspartate receptors". The Journal of Neuroscience. 33 (44): 17290–17300. doi:10.1523/JNEUROSCI.2619-13.2013. PMC 3812502. PMID 24174662.
- Hawkes CH, Doty RL (12 February 2009). The Neurology of Olfaction. Cambridge University Press. pp. 37–. ISBN 978-0-521-68216-9.
- ^ Monti-Bloch L, Jennings-White C, Dolberg DS, Berliner DL (1994). "The human vomeronasal system". Psychoneuroendocrinology. 19 (5–7): 673–86. doi:10.1016/0306-4530(94)90049-3. PMID 7938363. S2CID 36129626.
- ^ Liebowitz MR, Salman E, Nicolini H, Rosenthal N, Hanover R, Monti L (June 2014). "Effect of an acute intranasal aerosol dose of PH94B on social and performance anxiety in women with social anxiety disorder". The American Journal of Psychiatry. 171 (6): 675–82. doi:10.1176/appi.ajp.2014.12101342. PMID 24700254. S2CID 38510058.
- Marx CE, Bradford DW, Hamer RM, Naylor JC, Allen TB, Lieberman JA, Strauss JL, Kilts JD (September 2011). "Pregnenolone as a novel therapeutic candidate in schizophrenia: emerging preclinical and clinical evidence". Neuroscience. 191: 78–90. doi:10.1016/j.neuroscience.2011.06.076. PMID 21756978. S2CID 26396652.
- Baulieu E, Schumacher M (2000). "Progesterone as a neuroactive neurosteroid, with special reference to the effect of progesterone on myelination". Steroids. 65 (10–11): 605–12. doi:10.1016/s0039-128x(00)00173-2. PMID 11108866. S2CID 14952168.
- Thomas P, Pang Y (2012). "Membrane progesterone receptors: evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells". Neuroendocrinology. 96 (2): 162–71. doi:10.1159/000339822. PMC 3489003. PMID 22687885.
- Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A (September 2006). "Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis". Proceedings of the National Academy of Sciences of the United States of America. 103 (39): 14602–7. Bibcode:2006PNAS..10314602A. doi:10.1073/pnas.0606544103. PMC 1600006. PMID 16984997.
- Mellon SH, Griffin LD (2002). "Neurosteroids: biochemistry and clinical significance". Trends in Endocrinology and Metabolism. 13 (1): 35–43. doi:10.1016/S1043-2760(01)00503-3. PMID 11750861. S2CID 11605131.
- Benarroch EE (March 2007). "Neurosteroids: endogenous modulators of neuronal excitability and plasticity". Neurology. 68 (12): 945–7. doi:10.1212/01.wnl.0000257836.09570.e1. PMID 17372131. S2CID 219216099.
- Vallée M, Mayo W, Koob GF, Le Moal M (2001). "Neurosteroids in learning and memory processes". International Review of Neurobiology. 46: 273–320. doi:10.1016/s0074-7742(01)46066-1. ISBN 9780123668462. PMID 11599303.
- ^ Engel SR, Grant KA (2001). "Neurosteroids and behavior". International Review of Neurobiology. 46: 321–48. doi:10.1016/S0074-7742(01)46067-3. ISBN 9780123668462. PMID 11599304.
- ^ King SR (2008). "Emerging roles for neurosteroids in sexual behavior and function". Journal of Andrology. 29 (5): 524–33. doi:10.2164/jandrol.108.005660. PMID 18567641.
- Joshi S, Rajasekaran K, Kapur J (June 2013). "GABAergic transmission in temporal lobe epilepsy: the role of neurosteroids". Experimental Neurology. 244: 36–42. doi:10.1016/j.expneurol.2011.10.028. PMC 3319002. PMID 22101060.
- ^ Girdler SS, Klatzkin R (October 2007). "Neurosteroids in the context of stress: implications for depressive disorders". Pharmacology & Therapeutics. 116 (1): 125–39. doi:10.1016/j.pharmthera.2007.05.006. PMC 2650267. PMID 17597217.
- ^ Bali A, Jaggi AS (January 2014). "Multifunctional aspects of allopregnanolone in stress and related disorders". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 48: 64–78. doi:10.1016/j.pnpbp.2013.09.005. PMID 24044974. S2CID 21399549.
- Gunn BG, Cunningham L, Mitchell SG, Swinny JD, Lambert JJ, Belelli D (January 2015). "GABAA receptor-acting neurosteroids: a role in the development and regulation of the stress response". Frontiers in Neuroendocrinology. 36: 28–48. doi:10.1016/j.yfrne.2014.06.001. PMC 4349499. PMID 24929099.
- ^ Bäckström T, Andersson A, Andreé L, Birzniece V, Bixo M, Björn I, Haage D, Isaksson M, Johansson IM, Lindblad C, Lundgren P, Nyberg S, Odmark IS, Strömberg J, Sundström-Poromaa I, Turkmen S, Wahlström G, Wang M, Wihlbäck AC, Zhu D, Zingmark E (December 2003). "Pathogenesis in menstrual cycle-linked CNS disorders". Annals of the New York Academy of Sciences. 1007 (1): 42–53. Bibcode:2003NYASA1007...42B. doi:10.1196/annals.1286.005. PMID 14993039. S2CID 20995334.
- Guille C, Spencer S, Cavus I, Epperson CN (July 2008). "The role of sex steroids in catamenial epilepsy and premenstrual dysphoric disorder: implications for diagnosis and treatment". Epilepsy & Behavior. 13 (1): 12–24. doi:10.1016/j.yebeh.2008.02.004. PMC 4112568. PMID 18346939.
- Finocchi C, Ferrari M (May 2011). "Female reproductive steroids and neuronal excitability". Neurological Sciences. 32 (Suppl 1): S31-5. doi:10.1007/s10072-011-0532-5. PMID 21533709. S2CID 8885335.
- Genazzani AR, Bernardi F, Monteleone P, Luisi S, Luisi M (2000). "Neuropeptides, neurotransmitters, neurosteroids, and the onset of puberty". Annals of the New York Academy of Sciences. 900 (1): 1–9. Bibcode:2000NYASA.900....1G. doi:10.1111/j.1749-6632.2000.tb06210.x. PMID 10818386. S2CID 19302118.
- Melcangi RC, Panzica G, Garcia-Segura LM (September 2011). "Neuroactive steroids: focus on human brain". Neuroscience. 191: 1–5. doi:10.1016/j.neuroscience.2011.06.024. hdl:10261/61590. PMID 21704130. S2CID 55704799.
- Andréen L, Sundström-Poromaa I, Bixo M, Nyberg S, Bäckström T (August 2006). "Allopregnanolone concentration and mood--a bimodal association in postmenopausal women treated with oral progesterone". Psychopharmacology. 187 (2): 209–21. doi:10.1007/s00213-006-0417-0. PMID 16724185. S2CID 1933116.
- ^ Bäckström T, Haage D, Löfgren M, Johansson IM, Strömberg J, Nyberg S, Andréen L, Ossewaarde L, van Wingen GA, Turkmen S, Bengtsson SK (September 2011). "Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons". Neuroscience. 191: 46–54. doi:10.1016/j.neuroscience.2011.03.061. PMID 21600269. S2CID 38928854.
- ^ Andréen L, Nyberg S, Turkmen S, van Wingen G, Fernández G, Bäckström T (September 2009). "Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABAA modulators". Psychoneuroendocrinology. 34 (8): 1121–32. doi:10.1016/j.psyneuen.2009.02.003. PMID 19272715. S2CID 22259026.
- ^ Bäckström T, Bixo M, Johansson M, Nyberg S, Ossewaarde L, Ragagnin G, Savic I, Strömberg J, Timby E, van Broekhoven F, van Wingen G (February 2014). "Allopregnanolone and mood disorders". Progress in Neurobiology. 113: 88–94. doi:10.1016/j.pneurobio.2013.07.005. PMID 23978486. S2CID 207407084.
- Maurice T, Urani A, Phan VL, Romieu P (2001). "The interaction between neuroactive steroids and the sigma1 receptor function: behavioral consequences and therapeutic opportunities". Brain Res. Brain Res. Rev. 37 (1–3): 116–32. doi:10.1016/s0165-0173(01)00112-6. PMID 11744080. S2CID 44931783.
- ^ Su TP, London ED, Jaffe JH (1988). "Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems". Science. 240 (4849): 219–21. Bibcode:1988Sci...240..219S. doi:10.1126/science.2832949. PMID 2832949.
- ^ Takebayashi M, Hayashi T, Su TP (2004). "A perspective on the new mechanism of antidepressants: neuritogenesis through sigma-1 receptors". Pharmacopsychiatry. 37 (Suppl 3): S208–13. doi:10.1055/s-2004-832679. PMID 15547787. S2CID 260243232.
- Rogawski MA, Reddy DS, 2004. Neurosteroids: endogenous modulators of seizure susceptibility. In: Rho, J.M., Sankar, R., Cavazos, J. (Eds.), Epilepsy: Scientific Foundations of Clinical Practice. Marcel Dekker, New York, 2004;319-355.
- Kokate TG, Yamaguchi S, Pannell LK, Rajamani U, Carroll DM, Grossman AB, Rogawski MA (November 1998). "Lack of anticonvulsant tolerance to the neuroactive steroid pregnanolone in mice". The Journal of Pharmacology and Experimental Therapeutics. 287 (2): 553–8. PMID 9808680.
- Reddy DS, Rogawski MA (December 2000). "Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself". The Journal of Pharmacology and Experimental Therapeutics. 295 (3): 1241–8. PMID 11082461.
- Devinsky O, Schachter S, Pacia S (1 January 2005). Complementary and Alternative Therapies for Epilepsy. Demos Medical Publishing. pp. 378–. ISBN 978-1-934559-08-6.
- "Brexanolone - Sage Therapeutics". AdisInsight. Springer Nature Switzerland AG.
- Griebel G, Holmes A (September 2013). "50 years of hurdles and hope in anxiolytic drug discovery" (PDF). Nature Reviews. Drug Discovery. 12 (9): 667–87. doi:10.1038/nrd4075. PMC 4176700. PMID 23989795.
- "Pregnenolone methyl ether - Mapreg". AdisInsight. Springer Nature Switzerland AG.
- Duchossoy Y, David S, Baulieu EE, Robel P (2011). "Treatment of experimental spinal cord injury with 3β-methoxy-pregnenolone". Brain Res. 1403: 57–66. doi:10.1016/j.brainres.2011.05.065. PMID 21704982. S2CID 42657539.
- Bianchi M, Baulieu EE (2012). "3β-Methoxy-pregnenolone (MAP4343) as an innovative therapeutic approach for depressive disorders". Proc. Natl. Acad. Sci. U.S.A. 109 (5): 1713–8. Bibcode:2012PNAS..109.1713B. doi:10.1073/pnas.1121485109. PMC 3277154. PMID 22307636.
- Baulieu ÉÉ (2015). "From steroid hormones to depressive states and senile dementias: New mechanistic, therapeutical and predictive approaches". Comptes Rendus Biologies. 338 (8–9): 613–6. doi:10.1016/j.crvi.2015.06.003. PMID 26251072.
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A (March 1998). "Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine". Proceedings of the National Academy of Sciences of the United States of America. 95 (6): 3239–44. Bibcode:1998PNAS...95.3239U. doi:10.1073/pnas.95.6.3239. PMC 19726. PMID 9501247.
- Pinna G, Costa E, Guidotti A (24 January 2006). "Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake". Psychopharmacology. 186 (3): 362–72. doi:10.1007/s00213-005-0213-2. PMID 16432684. S2CID 7799814.
- ^ Reddy, Doodipala Samba (2010). "Neurosteroids". Sex Differences in the Human Brain, their Underpinnings and Implications. Progress in Brain Research. Vol. 186. pp. 113–137. doi:10.1016/B978-0-444-53630-3.00008-7. ISBN 978-0-444-53630-3. ISSN 0079-6123. PMC 3139029. PMID 21094889.
- Griffin LD, Mellon SH (November 1999). "Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes". Proc. Natl. Acad. Sci. U.S.A. 96 (23): 13512–7. Bibcode:1999PNAS...9613512G. doi:10.1073/pnas.96.23.13512. PMC 23979. PMID 10557352.
- Pinna G (September 2010). "In a mouse model relevant for post-traumatic stress disorder, selective brain steroidogenic stimulants (SBSS) improve behavioral deficits by normalizing allopregnanolone biosynthesis". Behav Pharmacol. 21 (5–6): 438–50. doi:10.1097/FBP.0b013e32833d8ba0. PMC 2942072. PMID 20716970.
- Schüle C, Romeo E, Uzunov DP, Eser D, di Michele F, Baghai TC, Pasini A, Schwarz M, Kempter H, Rupprecht R (March 2006). "Influence of mirtazapine on plasma concentrations of neuroactive steroids in major depression and on 3alpha-hydroxysteroid dehydrogenase activity". Mol. Psychiatry. 11 (3): 261–72. doi:10.1038/sj.mp.4001782. PMID 16344854. S2CID 21473462.
- Dhir A, Rogawski MA (April 2012). "Role of neurosteroids in the anticonvulsant activity of midazolam". British Journal of Pharmacology. 165 (8): 2684–91. doi:10.1111/j.1476-5381.2011.01733.x. PMC 3423249. PMID 22014182.
- Wang, Mingde (2011). "Neurosteroids and GABA-A Receptor Function". Frontiers in Endocrinology. 2: 44. doi:10.3389/fendo.2011.00044. ISSN 1664-2392. PMC 3356040. PMID 22654809.
- Usami N, Yamamoto T, Shintani S, Ishikura S, Higaki Y, Katagiri Y, Hara A (April 2002). "Substrate specificity of human 3(20)alpha-hydroxysteroid dehydrogenase for neurosteroids and its inhibition by benzodiazepines" (pdf). Biological & Pharmaceutical Bulletin. 25 (4): 441–5. doi:10.1248/bpb.25.441. PMID 11995921.
Further reading
- Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, Mennerick S (December 2005). "Neurosteroid access to the GABAA receptor". The Journal of Neuroscience. 25 (50): 11605–13. doi:10.1523/JNEUROSCI.4173-05.2005. PMC 6726021. PMID 16354918.
- Wang JM, Johnston PB, Ball BG, Brinton RD (May 2005). "The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression". The Journal of Neuroscience. 25 (19): 4706–18. doi:10.1523/JNEUROSCI.4520-04.2005. PMC 6724768. PMID 15888646.
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, Watanabe H, Costa E, Guidotti A (February 2001). "Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation". Proceedings of the National Academy of Sciences of the United States of America. 98 (5): 2849–54. Bibcode:2001PNAS...98.2849D. doi:10.1073/pnas.051628598. PMC 30228. PMID 11226329.
- Melcangi RC, Celotti F, Martini L (March 1994). "Progesterone 5-alpha-reduction in neuronal and in different types of glial cell cultures: type 1 and 2 astrocytes and oligodendrocytes". Brain Research. 639 (2): 202–6. doi:10.1016/0006-8993(94)91731-0. PMID 8205473. S2CID 37105244.
- Corpéchot C, Robel P, Axelson M, Sjövall J, Baulieu EE (August 1981). "Characterization and measurement of dehydroepiandrosterone sulfate in rat brain". Proceedings of the National Academy of Sciences of the United States of America. 78 (8): 4704–7. Bibcode:1981PNAS...78.4704C. doi:10.1073/pnas.78.8.4704. PMC 320231. PMID 6458035.
- Reddy D, Rogawski MA (2012). "Neurosteroids — Endogenous regulators of seizure susceptibility and role in the treatment of epilepsy". In Noebels JL, Avoli M, Rogawski MA, et al. (eds.). Jasper's Basic Mechanisms of the Epilepsies (4th ed.). Bethesda (MD): National Center for Biotechnology Information. PMID 22787590.
| General anesthetics (N01A) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhalational | |||||||||||||||
| Injection |
| ||||||||||||||
| |||||||||||||||
Categories: