| |
| Names | |
|---|---|
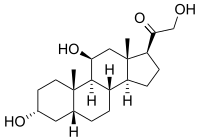
| IUPAC name 3α,11β,21-Trihydroxy-5β-pregnan-20-one | |
| Systematic IUPAC name 1-phenanthren-1-yl]-2-hydroxyethan-1-one | |
| Other names 3α,5α-Tetrahydrocorticosterone; 5β-Pregnane-3α,11β,21-triol-20-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.000.627 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C21H34O4 |
| Molar mass | 350.499 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
3α,5α-Tetrahydrocorticosterone (3α,5α-THB), or simply tetrahydrocorticosterone (THB or THCC), is an endogenous glucocorticoid hormone.
See also
- 5α-Dihydrocorticosterone
- Tetrahydrodeoxycorticosterone
- Dihydrodeoxycorticosterone
- Allopregnanolone
- Tetrahydrocortisone
- Tetrahydrocortisol
References
- McInnes KJ, Kenyon CJ, Chapman KE, et al. (May 2004). "5alpha-reduced glucocorticoids, novel endogenous activators of the glucocorticoid receptor". The Journal of Biological Chemistry. 279 (22): 22908–12. doi:10.1074/jbc.M402822200. PMID 15044432.
| Glucocorticoid receptor modulators | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| GRTooltip Glucocorticoid receptor |
| ||||||||
This article about a steroid is a stub. You can help Misplaced Pages by expanding it. |