| |
| Names | |
|---|---|
| Preferred IUPAC name 2,2′,2′′-Nitrilotriacetic acid | |
| Other names
N,N-Bis(carboxymethyl)glycine 2-acetic acid Triglycine Trilon | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 1710776 |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.004.869 |
| EC Number |
|
| Gmelin Reference | 3726 |
| KEGG | |
| MeSH | Nitrilotriacetic+Acid |
| PubChem CID | |
| RTECS number |
|
| UNII |
|
| UN number | 2811 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
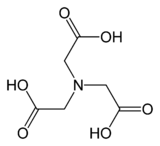
| Chemical formula | C6H9NO6 |
| Molar mass | 191.14 |
| Appearance | White crystals |
| Melting point | 246 °C (475 °F; 519 K) |
| Solubility in water | Insoluble. <0.01 g/100 mL at 23°C |
| Thermochemistry | |
| Std enthalpy of formation (ΔfH298) |
−1.3130–−1.3108 MJ mol |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Warning |
| Hazard statements | H302, H319, H351 |
| Precautionary statements | P281, P305+P351+P338 |
| Flash point | 100 °C (212 °F; 373 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 1.1 g kg (oral, rat) |
| Related compounds | |
| Related alkanoic acids | |
| Related compounds | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
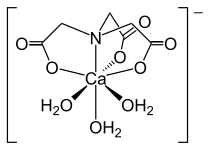
Nitrilotriacetic acid (NTA) is the aminopolycarboxylic acid with the formula N(CH2CO2H)3. It is a colourless solid. Its conjugate base nitrilotriacetate is used as a chelating agent for Ca, Co, Cu, and Fe.
Production and use
Nitrilotriacetic acid is commercially available as the free acid and as the sodium salt. It is produced from ammonia, formaldehyde, and sodium cyanide or hydrogen cyanide. Worldwide capacity is estimated at 100 thousand tonnes per year. NTA is also cogenerated as an impurity in the synthesis of EDTA, arising from reactions of the ammonia coproduct. Older routes to NTA included alkylation of ammonia with chloroacetic acid and oxidation of triethanolamine.
Coordination chemistry and applications
The conjugate base of NTA is a tripodal tetradentate trianionic ligand, forming coordination compounds with a variety of metal ions.
Like EDTA, its sodium salt is used for water softening to remove Ca. For this purpose, NTA is a replacement for triphosphate, which once was widely used in detergents, and cleansers, but can cause eutrophication of lakes.
In one application, sodium NTA removes Cr, Cu, and As from wood that had been treated with chromated copper arsenate.
Laboratory uses
In the laboratory, this compound is used in complexometric titrations. A variant of NTA is used for protein isolation and purification in the His-tag method. The modified NTA is used to immobilize nickel on a solid support. This allows purification of proteins containing a tag consisting of six histidine residues at either terminus.
The His-tag binds the metal of metal chelator complexes. Previously, iminodiacetic acid was used for that purpose. Now, nitrilotriacetic acid is more commonly used.
For laboratory uses, Ernst Hochuli et al. (1987) coupled the NTA ligand and nickel ions to agarose beads. This Ni-NTA Agarose is the most used tool to purify His-tagged proteins via affinity chromatography.
Toxicity and environment
In contrast to EDTA, NTA is easily biodegradable and is almost completely removed during wastewater treatment. The environmental impacts of NTA are minimal. Despite widespread use in cleaning products, the concentration in the water supply is too low to have a sizeable impact on human health or environmental quality.
Related compounds
- N-Methyliminodiacetic acid (MIDA), the N-methyl derivative of IDA
- Imidodiacetic acid, the amino diacetic acid
- N-(2-Carboxyethyl)iminodiacetic acid, a more biodegradable analogue of NTA
- N-hydroxyiminodiacetic acid (HIDA), HON(CH2CO2H)2 (registry number = 87339-38-6) See HIDA scan.
References
- "Nitrilotriacetic Acid - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification. Retrieved 13 July 2012.
- Nitrilotriacetic acid
- Favre, Henri A.; Powell, Warren H. (2014). Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. pp. 21, 679. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ ChemBK Chemical Database http://www.chembk.com/en/chem/Nitrilotriacetic%20acid
- Nitrilotriacetic Acid and Its Salts, International Agency for Research on Cancer
- ^ Thomas Schmidt, Charalampos Gousetis, Hans-Joachim Opgenorth (2022). "Nitrilotriacetic Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_377.pub3. ISBN 978-3527306732.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link) - Hart, J. Roger (2005) "Ethylenediaminetetraacetic Acid and Related Chelating Agents" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. doi:10.1002/14356007.a10_095
- B. L. Barnett, V. A. Uchtman (1979). "Structural Investigations of Calcium-Binding Molecules. 4. Calcium Binding to Aminocarboxylates. Crystal Structures of Ca(CaEDTA)7H2O and Na(CaNTA)". Inorg. Chem. 18 (10): 2674–2678. doi:10.1021/ic50200a007.
- Fang-Chih, C.; Ya-Nang, W.; Pin-Jui, C.; Chun-Han, K. Factors affecting chelating extraction of Cr, Cu, and As from CCA-treated wood. J. Environ. Manag. 2013, 122.
- Liu, Weijing (2016). "Layer-by-Layer Deposition with Polymers Containing Nitrilotriacetate, A Convenient Route to Fabricate Metal- and Protein-Binding Films". ACS Applied Materials & Interfaces. 8 (16): 10164–73. doi:10.1021/acsami.6b00896. PMID 27042860.
- qiaexpressionist
- Lauer, Sabine A.; Nolan, John P. (2002). "Development and characterization of Ni-NTA-bearing microspheres". Cytometry. 48 (3): 136–145. doi:10.1002/cyto.10124. ISSN 1097-0320. PMID 12116359.
- Hochuli, E.; Döbeli, H.; Schacher, A. (January 1987). "New metal chelate adsorbent selective for proteins and peptides containing neighbouring histidine residues". Journal of Chromatography A. 411: 177–184. doi:10.1016/s0021-9673(00)93969-4. ISSN 0021-9673. PMID 3443622.
- Brouwer, N.; Terpstra, P. Ecological and Toxicological Properties of Nitrilotriacetic Acid (NTA) as a Detergent Builder. Tenside Surfactants Detergents 1995, 32, 225-228.
- Hubregtse, Ton; Hanefeld, Ulf; Arends, Isabel W. C. E. (2007). "Stabilizing Factors for Vanadium(IV) in Amavadin". European Journal of Organic Chemistry. 2007 (15): 2413–2422. doi:10.1002/ejoc.200601053.