Nitrogen-15 nuclear magnetic resonance spectroscopy (nitrogen-15 NMR spectroscopy, or just simply N NMR) is a version of nuclear magnetic resonance spectroscopy that examines samples containing the N nucleus. N NMR differs in several ways from the more common C and H NMR. To circumvent the difficulties associated with measurement of the quadrupolar, spin-1 N nuclide, N NMR is employed in samples for detection since it has a ground-state spin of ½. SinceN is 99.64% abundant, incorporation of N into samples often requires novel synthetic techniques.
Nitrogen-15 is frequently used in nuclear magnetic resonance spectroscopy (NMR), because unlike the more abundant nitrogen-14, that has an integer nuclear spin and thus a quadrupole moment, N has a fractional nuclear spin of one-half, which offers advantages for NMR like narrower line width. Proteins can be isotopically labeled by cultivating them in a medium containing nitrogen-15 as the only source of nitrogen. In addition, nitrogen-15 is used to label proteins in quantitative proteomics (e.g. SILAC).
Implementation
N NMR has complications not encountered in H and C NMR spectroscopy. The 0.36% natural abundance of N results in a major sensitivity penalty. Sensitivity is made worse by its low gyromagnetic ratio (γ = −27.126 × 10 Ts), which is 10.14% that of H. The signal-to-noise ratio for H is about 300-fold greater than N at the same magnetic field strength.
Physical properties
The physical properties of N are quite different from other nuclei. Its properties along with several common nuclei are summarized in the below table.
| Isotope | Magnetic dipole moment (μN) |
Nuclear spin number |
Natural abundance (%) |
Gyromagnetic ratio (10 rad s T) |
NMR frequency at 11.7T (MHz) |
|---|---|---|---|---|---|
| H | 2.79284734(3) | 1/2 | ~100 | 267.522 | -500 |
| H | 0.857438228(9) | 1 | 0.015 | 41.066 | -76.753 |
| H | 2.97896244(4) | 1/2 | 0 | 285.349 | -533.32 |
| B | 1.80064478(6) | 3 | 19.9 | 28.747 | -53.718 |
| B | 2.6886489 | 3/2 | 80.1 | 85.847 | -160.42 |
| C | 0.7024118(14) | 1/2 | 1.1 | 67.238 | -125.725 |
| N | 0.40376100(6) | 1 | 99.6 | 19.338 | -36.132 |
| N | -0.28318884(5) | 1/2 | 0.37 | -27.126 | 50.782 |
| O | -1.89379(9) | 5/2 | 0.04 | -36.281 | 67.782 |
| F | 2.628868(8) | 1/2 | ~100 | 251.815 | -470.47 |
| P | 1.13160(3) | 1/2 | ~100 | 108.394 | -202.606 |
From these data, one can see that at full enrichment, N is about one tenth (-27.126/267.522) as sensitive as H.
Chemical shift trends
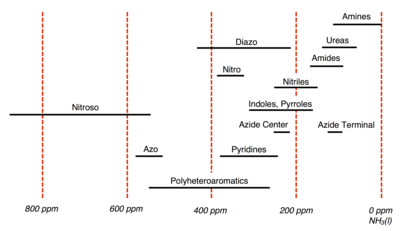
The International Union of Pure and Applied Chemistry (IUPAC) recommends using CH3NO2 as the experimental standard; however in practice many spectroscopists utilize pressurized NH3(l) instead. For N, chemical shifts referenced with NH3(l) are 380.5 ppm upfield from CH3NO2 (δNH3 = δCH3NO2 + 380.5 ppm). Chemical shifts for N are somewhat erratic but typically they span a range of -400 ppm to 1100 ppm with respect to CH3NO2. Below is a summary of N chemical shifts for common organic groups referenced with respect to NH3, whose chemical shift is assigned 0 ppm.
Gyromagnetic ratio

Unlike most nuclei, the gyromagnetic ratio for N is negative. With the spin precession phenomenon, the sign of γ determines the sense (clockwise vs counterclockwise) of precession. Most common nuclei have positive gyromagnetic ratios such as H and C.
Applications
Tautomerization

N NMR is used in a wide array of areas from biological to inorganic techniques. A famous application in organic synthesis is to utilize N to monitor tautomerization equilibria in heteroaromatics because of the dramatic change in N shifts between tautomers.
Protein NMR
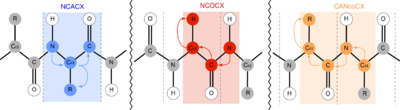
N NMR is also extremely valuable in protein NMR investigations. Most notably, the introduction of three-dimensional experiments with N lifts the ambiguity in C–C two-dimensional experiments. In solid-state nuclear magnetic resonance (ssNMR), for example, N is most commonly utilized in NCACX, NCOCX, and CANcoCX pulse sequences.
Investigation of nitrogen-containing heterocycles
N NMR is the most effective method for investigation of structure of heterocycles with a high content of nitrogen atoms (tetrazoles, triazines and their annelated analogs). N labeling followed by analysis of C–N and H–N couplings may be used for establishing structures and chemical transformations of nitrogen heterocycles.
INEPT

Insensitive nuclei enhanced by polarization transfer (INEPT) is a signal resolution enhancement method. Because N has a gyromagnetic ratio that is small in magnitude, the resolution is quite poor. A common pulse sequence which dramatically improves the resolution for N is INEPT. The INEPT is an elegant solution in most cases because it increases the Boltzmann polarization and lowers T1 values (thus scans are shorter). Additionally, INEPT can accommodate negative gyromagnetic ratios, whereas the common nuclear Overhauser effect (NOE) cannot.
See also
- Heteronuclear single quantum coherence spectroscopy (HSQC)
- Two-dimensional nuclear magnetic resonance spectroscopy
- Triple-resonance nuclear magnetic resonance spectroscopy
References
- ^ Witanowski, M (1974). “Nitrogen N.M.R. Spectroscopy”. Pure and Applied Chemistry. 37, pp. 225-233. doi:10.1351/pac197437010225
- ^ J. H. Nelson (2003). Nuclear Magnetic Resonance Spectroscopy. Prentice-Hall. ISBN 978-0130334510.
- ^ M H Levitt (2008). Spin Dynamics. John Wiley & Sons Ltd. ISBN 978-0470511176.
- ^ Arthur G Palmer (2007). Protein NMR Spectroscopy. Elsevier Academic Press. ISBN 978-0121644918.
- Stone, Nicholas J (2005). "Table of nuclear magnetic dipole and electric quadrupole moments". Atomic Data and Nuclear Data Tables. 90 (1), pp. 75-176. doi:10.1016/j.adt.2005.04.001
- ^ Mooney, E F; Winson, P H (1969). “Nitrogen Magnetic Resonance Spectroscopy”. Annual Reports on NMR Spectroscopy (2), pp 125-152. doi:10.1016/S0066-4103(08)60321-X
- Shestakova, Tatyana S.; Shenkarev, Zakhar O.; Deev, Sergey L.; Chupakhin, Oleg N.; Khalymbadzha, Igor A.; Rusinov, Vladimir L.; Arseniev, Alexander S. (2013-06-27). "Long-Range 1H–15N J Couplings Providing a Method for Direct Studies of the Structure and Azide–Tetrazole Equilibrium in a Series of Azido-1,2,4-triazines and Azidopyrimidines" (PDF). The Journal of Organic Chemistry. 78 (14): 6975–6982. doi:10.1021/jo4008207. hdl:10995/27205. ISSN 0022-3263. PMID 23751069.
- Deev, Sergey L; Paramonov, Alexander S; Shestakova, Tatyana S; Khalymbadzha, Igor A; Chupakhin, Oleg N; Subbotina, Julia O; Eltsov, Oleg S; Slepukhin, Pavel A; Rusinov, Vladimir L (2017-11-29). "15N-Labelling and structure determination of adamantylated azolo-azines in solution". Beilstein Journal of Organic Chemistry. 13 (1): 2535–2548. doi:10.3762/bjoc.13.250. ISSN 1860-5397. PMC 5727827. PMID 29259663.
- Deev, Sergey L.; Khalymbadzha, Igor A.; Shestakova, Tatyana S.; Charushin, Valery N.; Chupakhin, Oleg N. (2019-08-23). "15 N labeling and analysis of 13C–15N and 1H–15N couplings in studies of the structures and chemical transformations of nitrogen heterocycles". RSC Advances. 9 (46): 26856–26879. Bibcode:2019RSCAd...926856D. doi:10.1039/C9RA04825A. ISSN 2046-2069. PMC 9070671. PMID 35528595.
| NMR spectroscopy by isotope | |
|---|---|
| Isotope | |