 | |
| Clinical data | |
|---|---|
| Dependence liability | High (same oxycodone) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.055.334 |
| Chemical and physical data | |
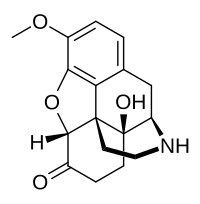
| Formula | C17H19NO4 |
| Molar mass | 301.342 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Noroxycodone is the major metabolite of the opioid analgesic oxycodone. It is formed from oxycodone in the liver via N-demethylation predominantly by CYP3A4. Noroxycodone binds to and activates the μ-opioid receptor (MOR) similarly to oxycodone, although with one-third of the affinity of oxycodone and 5- to 10-fold lower activational potency. However, although a potent MOR agonist, noroxycodone poorly crosses the blood-brain-barrier into the central nervous system, and for this reason, is only minimally analgesic in comparison.
See also
References
- ^ Smith HS, Vanderah TW, McClean G (25 April 2008). "Opioids for Pain". In Smith H, Passik S (eds.). Pain and Chemical Dependency. Oxford University Press, USA. pp. 195–. ISBN 978-0-19-530055-0.
- ^ Pincus MR, Bluth MH, Abraham Jr NZ (31 March 2016). "Toxicology and Therapeutic Drug Monitoring". In McPherson RA, Pincus MR (eds.). Henry's Clinical Diagnosis and Management by Laboratory Methods. Elsevier Health Sciences. pp. 336–. ISBN 978-0-323-41315-2.
- ^ Somogyi AA, Coller JK (29 May 2012). "Drugs against Acute and Chronic Pain". In Anzenbacher P, Zanger UM (eds.). Metabolism of Drugs and Other Xenobiotics. John Wiley & Sons. pp. 420–. ISBN 978-3-527-32903-8.
- ^ Lemberg KK, Siiskonen AO, Kontinen VK, Yli-Kauhaluoma JT, Kalso EA (February 2008). "Pharmacological characterization of noroxymorphone as a new opioid for spinal analgesia". Anesthesia and Analgesia. 106 (2): 463–70, table of contents. doi:10.1213/ane.0b013e3181605a15. PMID 18227301. S2CID 16524280.
- ^ Kokki H, Kokki M (25 April 2016). "Central Nervous System Penetration of the Opioid Oxycodone". In Preedy VR (ed.). Neuropathology of Drug Addictions and Substance Misuse Volume 3: General Processes and Mechanisms, Prescription Medications, Caffeine and Areca, Polydrug Misuse, Emerging Addictions and Non-Drug Addictions. Elsevier Science. pp. 462–464. ISBN 978-0-12-800677-1.
- Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, Shen DD (May 2006). "Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites". Clinical Pharmacology and Therapeutics. 79 (5): 461–479. doi:10.1016/j.clpt.2006.01.009. PMID 16678548. S2CID 21372271.
- Klimas R, Witticke D, El Fallah S, Mikus G (May 2013). "Contribution of oxycodone and its metabolites to the overall analgesic effect after oxycodone administration". Expert Opinion on Drug Metabolism & Toxicology. 9 (5): 517–528. doi:10.1517/17425255.2013.779669. PMID 23488585. S2CID 22857902.
This analgesic-related article is a stub. You can help Misplaced Pages by expanding it. |