Retinoid X receptor alpha (RXR-alpha), also known as NR2B1 (nuclear receptor subfamily 2, group B, member 1) is a nuclear receptor that in humans is encoded by the RXRA gene.
Function
Retinoid X receptors (RXRs) and retinoic acid receptors (RARs), are nuclear receptors that mediate the biological effects of retinoids by their involvement in retinoic acid-mediated gene activation. These receptors exert their action by binding, as homodimers or heterodimers, to specific sequences in the promoters of target genes and regulating their transcription. The protein encoded by this gene is a member of the steroid and thyroid hormone receptor superfamily of transcription factors. In the absence of ligand, the RXR-RAR heterodimers associate with a multiprotein complex containing transcription corepressors that induce histone deacetylation, chromatin condensation and transcriptional suppression. On ligand binding, the corepressors dissociate from the receptors and associate with the coactivators leading to transcriptional activation. The RXRA/PPARA heterodimer is required for PPARA transcriptional activity on fatty acid oxidation genes such as ACOX1 and the cytochrome P450 system genes.
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles.
[[File: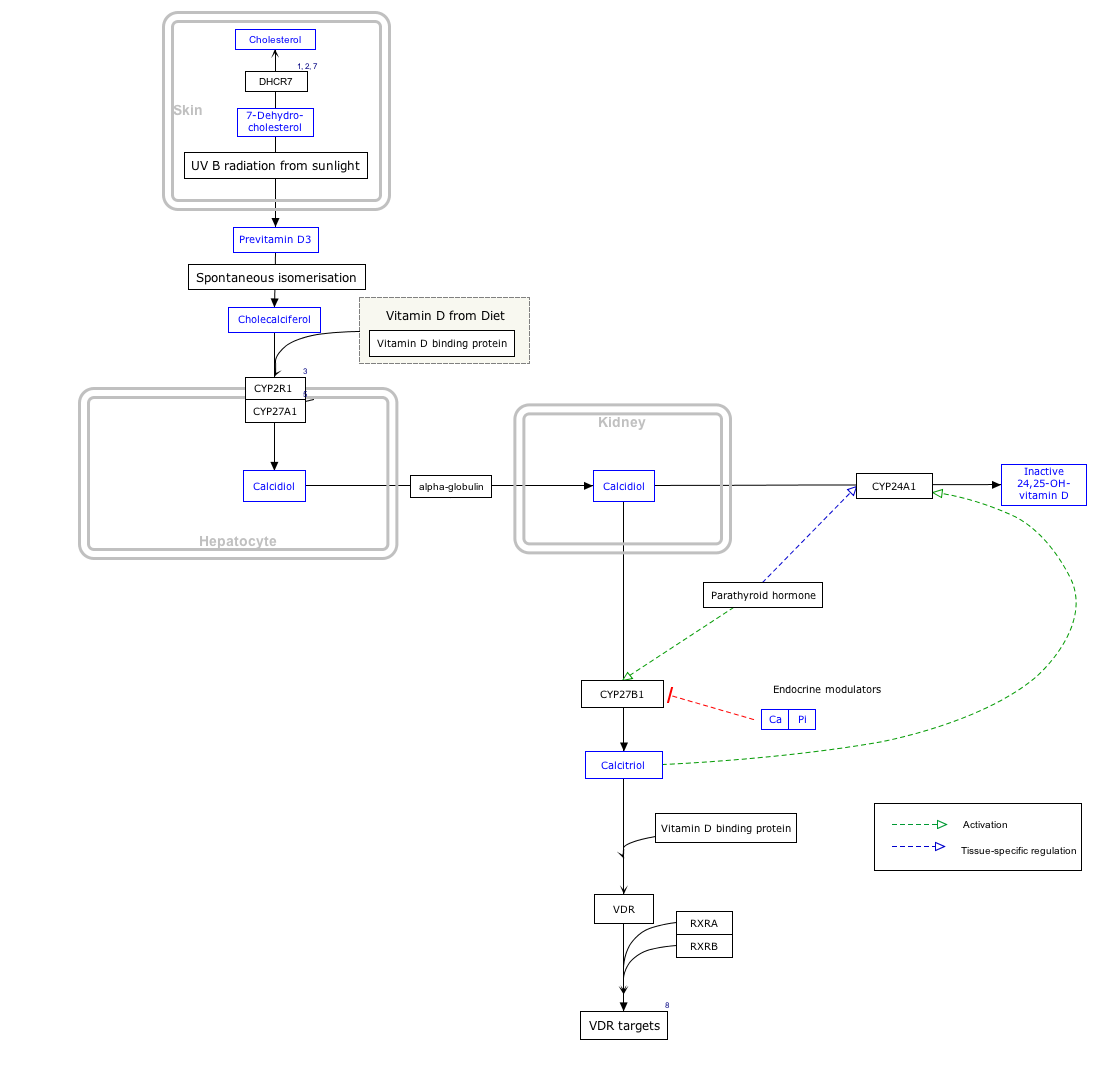

- The interactive pathway map can be edited at WikiPathways: "VitaminDSynthesis_WP1531".
Interactions
Retinoid X receptor alpha has been shown to interact with:
- BCL3,
- BRD8,
- CLOCK,
- FXR
- IGFBP3,
- ITGB3BP,
- LXR-β,
- MyoD,
- NCOA6,
- NFKBIB,
- NPAS2,
- NRIP1,
- NR4A1,
- NCOA2,
- NCOA3,
- POU2F1,
- PPARGC1A,
- PPAR-γ,
- RNF8,
- RAR-α,
- SHP,
- TADA3L,
- TBP,
- TRIM24,
- TR-β, and
- VDR.
See also
References
- ^ GRCh38: Ensembl release 89: ENSG00000186350 – Ensembl, May 2017
- ^ GRCm38: Ensembl release 89: ENSMUSG00000015846 – Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Mangelsdorf DJ, Ong ES, Dyck JA, Evans RM (May 1990). "Nuclear receptor that identifies a novel retinoic acid response pathway". Nature. 345 (6272): 224–9. Bibcode:1990Natur.345..224M. doi:10.1038/345224a0. PMID 2159111. S2CID 4232833.
- "Entrez Gene: RXRA retinoid X receptor, alpha".
- "Retinoic acid receptor RXR-alpha - Homo sapiens (Human)". UniProt.
- Na SY, Choi HS, Kim JW, Na DS, Lee JW (1998). "Bcl3, an IkappaB protein, as a novel transcription coactivator of the retinoid X receptor". J. Biol. Chem. 273 (47): 30933–8. doi:10.1074/jbc.273.47.30933. PMID 9812988.
- Monden T, Kishi M, Hosoya T, Satoh T, Wondisford FE, Hollenberg AN, Yamada M, Mori M (1999). "p120 acts as a specific coactivator for 9-cis-retinoic acid receptor (RXR) on peroxisome proliferator-activated receptor-gamma/RXR heterodimers". Mol. Endocrinol. 13 (10): 1695–703. doi:10.1210/mend.13.10.0353. PMID 10517671. S2CID 25098867.
- ^ McNamara P, Seo SB, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA (2001). "Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock". Cell. 105 (7): 877–89. doi:10.1016/S0092-8674(01)00401-9. PMID 11439184. S2CID 6251321.
- ^ Seol W, Choi HS, Moore DD (1995). "Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors". Mol. Endocrinol. 9 (1): 72–85. doi:10.1210/mend.9.1.7760852. PMID 7760852.
- Liu B, Lee HY, Weinzimer SA, Powell DR, Clifford JL, Kurie JM, Cohen P (2000). "Direct functional interactions between insulin-like growth factor-binding protein-3 and retinoid X receptor-alpha regulate transcriptional signaling and apoptosis". J. Biol. Chem. 275 (43): 33607–13. doi:10.1074/jbc.M002547200. PMID 10874028.
- Li D, Desai-Yajnik V, Lo E, Schapira M, Abagyan R, Samuels HH (1999). "NRIF3 is a novel coactivator mediating functional specificity of nuclear hormone receptors". Mol. Cell. Biol. 19 (10): 7191–202. doi:10.1128/mcb.19.10.7191. PMC 84712. PMID 10490654.
- Froeschlé A, Alric S, Kitzmann M, Carnac G, Auradé F, Rochette-Egly C, Bonnieu A (1998). "Retinoic acid receptors and muscle b-HLH proteins: partners in retinoid-induced myogenesis". Oncogene. 16 (26): 3369–78. doi:10.1038/sj.onc.1201894. PMID 9692544. S2CID 6667231.
- Lee SK, Anzick SL, Choi JE, Bubendorf L, Guan XY, Jung YK, Kallioniemi OP, Kononen J, Trent JM, Azorsa D, Jhun BH, Cheong JH, Lee YC, Meltzer PS, Lee JW (1999). "A nuclear factor, ASC-2, as a cancer-amplified transcriptional coactivator essential for ligand-dependent transactivation by nuclear receptors in vivo". J. Biol. Chem. 274 (48): 34283–93. doi:10.1074/jbc.274.48.34283. PMID 10567404.
- Lee SK, Jung SY, Kim YS, Na SY, Lee YC, Lee JW (2001). "Two distinct nuclear receptor-interaction domains and CREB-binding protein-dependent transactivation function of activating signal cointegrator-2". Mol. Endocrinol. 15 (2): 241–54. doi:10.1210/mend.15.2.0595. PMID 11158331.
- Kong HJ, Park MJ, Hong S, Yu HJ, Lee YC, Choi YH, Cheong J (2003). "Hepatitis B virus X protein regulates transactivation activity and protein stability of the cancer-amplified transcription coactivator ASC-2". Hepatology. 38 (5): 1258–66. doi:10.1053/jhep.2003.50451. PMID 14578865. S2CID 29349615.
- Ko L, Cardona GR, Iwasaki T, Bramlett KS, Burris TP, Chin WW (2002). "Ser-884 adjacent to the LXXLL motif of coactivator TRBP defines selectivity for ERs and TRs". Mol. Endocrinol. 16 (1): 128–40. doi:10.1210/mend.16.1.0755. PMID 11773444.
- Na SY, Kim HJ, Lee SK, Choi HS, Na DS, Lee MO, Chung M, Moore DD, Lee JW (1998). "IkappaBbeta interacts with the retinoid X receptor and inhibits retinoid-dependent transactivation in lipopolysaccharide-treated cells". J. Biol. Chem. 273 (6): 3212–5. doi:10.1074/jbc.273.6.3212. PMID 9452433.
- Farooqui M, Franco PJ, Thompson J, Kagechika H, Chandraratna RA, Banaszak L, Wei LN (2003). "Effects of retinoid ligands on RIP140: molecular interaction with retinoid receptors and biological activity". Biochemistry. 42 (4): 971–9. doi:10.1021/bi020497k. PMID 12549917.
- L'Horset F, Dauvois S, Heery DM, Cavaillès V, Parker MG (1996). "RIP-140 interacts with multiple nuclear receptors by means of two distinct sites". Mol. Cell. Biol. 16 (11): 6029–36. doi:10.1128/MCB.16.11.6029. PMC 231605. PMID 8887632.
- Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, Zhang XK (2004). "Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3". Cell. 116 (4): 527–40. doi:10.1016/S0092-8674(04)00162-X. PMID 14980220. S2CID 17808479.
- ^ Zhang C, Baudino TA, Dowd DR, Tokumaru H, Wang W, MacDonald PN (2001). "Ternary complexes and cooperative interplay between NCoA-62/Ski-interacting protein and steroid receptor coactivators in vitamin D receptor-mediated transcription". J. Biol. Chem. 276 (44): 40614–20. doi:10.1074/jbc.M106263200. PMID 11514567.
- Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM (1997). "Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300". Cell. 90 (3): 569–80. doi:10.1016/S0092-8674(00)80516-4. PMID 9267036. S2CID 15284825.
- Préfontaine GG, Walther R, Giffin W, Lemieux ME, Pope L, Haché RJ (1999). "Selective binding of steroid hormone receptors to octamer transcription factors determines transcriptional synergism at the mouse mammary tumor virus promoter". J. Biol. Chem. 274 (38): 26713–9. doi:10.1074/jbc.274.38.26713. PMID 10480874.
- Kakizawa T, Miyamoto T, Ichikawa K, Kaneko A, Suzuki S, Hara M, Nagasawa T, Takeda T, Mori Ji, Kumagai M, Hashizume K (1999). "Functional interaction between Oct-1 and retinoid X receptor". J. Biol. Chem. 274 (27): 19103–8. doi:10.1074/jbc.274.27.19103. PMID 10383413.
- Delerive P, Wu Y, Burris TP, Chin WW, Suen CS (2002). "PGC-1 functions as a transcriptional coactivator for the retinoid X receptors". J. Biol. Chem. 277 (6): 3913–7. doi:10.1074/jbc.M109409200. PMID 11714715.
- Tontonoz P, Graves RA, Budavari AI, Erdjument-Bromage H, Lui M, Hu E, Tempst P, Spiegelman BM (1994). "Adipocyte-specific transcription factor ARF6 is a heterodimeric complex of two nuclear hormone receptors, PPAR gamma and RXR alpha". Nucleic Acids Res. 22 (25): 5628–34. doi:10.1093/nar/22.25.5628. PMC 310126. PMID 7838715.
- Berger J, Patel HV, Woods J, Hayes NS, Parent SA, Clemas J, Leibowitz MD, Elbrecht A, Rachubinski RA, Capone JP, Moller DE (2000). "A PPARgamma mutant serves as a dominant negative inhibitor of PPAR signaling and is localized in the nucleus". Mol. Cell. Endocrinol. 162 (1–2): 57–67. doi:10.1016/S0303-7207(00)00211-2. PMID 10854698. S2CID 20343538.
- Gampe RT, Montana VG, Lambert MH, Miller AB, Bledsoe RK, Milburn MV, Kliewer SA, Willson TM, Xu HE (2000). "Asymmetry in the PPARgamma/RXRalpha crystal structure reveals the molecular basis of heterodimerization among nuclear receptors". Mol. Cell. 5 (3): 545–55. doi:10.1016/S1097-2765(00)80448-7. PMID 10882139.
- Takano Y, Adachi S, Okuno M, Muto Y, Yoshioka T, Matsushima-Nishiwaki R, Tsurumi H, Ito K, Friedman SL, Moriwaki H, Kojima S, Okano Y (2004). "The RING finger protein, RNF8, interacts with retinoid X receptor alpha and enhances its transcription-stimulating activity". J. Biol. Chem. 279 (18): 18926–34. doi:10.1074/jbc.M309148200. PMID 14981089.
- Benkoussa M, Brand C, Delmotte MH, Formstecher P, Lefebvre P (2002). "Retinoic acid receptors inhibit AP1 activation by regulating extracellular signal-regulated kinase and CBP recruitment to an AP1-responsive promoter". Mol. Cell. Biol. 22 (13): 4522–34. doi:10.1128/MCB.22.13.4522-4534.2002. PMC 133906. PMID 12052862.
- Bugge TH, Pohl J, Lonnoy O, Stunnenberg HG (1992). "RXR alpha, a promiscuous partner of retinoic acid and thyroid hormone receptors". EMBO J. 11 (4): 1409–18. doi:10.1002/j.1460-2075.1992.tb05186.x. PMC 556590. PMID 1314167.
- Lee YK, Dell H, Dowhan DH, Hadzopoulou-Cladaras M, Moore DD (2000). "The orphan nuclear receptor SHP inhibits hepatocyte nuclear factor 4 and retinoid X receptor transactivation: two mechanisms for repression". Mol. Cell. Biol. 20 (1): 187–95. doi:10.1128/MCB.20.1.187-195.2000. PMC 85074. PMID 10594021.
- Brendel C, Schoonjans K, Botrugno OA, Treuter E, Auwerx J (2002). "The small heterodimer partner interacts with the liver X receptor alpha and represses its transcriptional activity". Mol. Endocrinol. 16 (9): 2065–76. doi:10.1210/me.2001-0194. PMID 12198243.
- Zeng M, Kumar A, Meng G, Gao Q, Dimri G, Wazer D, Band H, Band V (2002). "Human papilloma virus 16 E6 oncoprotein inhibits retinoic X receptor-mediated transactivation by targeting human ADA3 coactivator". J. Biol. Chem. 277 (47): 45611–8. doi:10.1074/jbc.M208447200. PMID 12235159.
- Schulman IG, Chakravarti D, Juguilon H, Romo A, Evans RM (1995). "Interactions between the retinoid X receptor and a conserved region of the TATA-binding protein mediate hormone-dependent transactivation". Proc. Natl. Acad. Sci. U.S.A. 92 (18): 8288–92. Bibcode:1995PNAS...92.8288S. doi:10.1073/pnas.92.18.8288. PMC 41142. PMID 7667283.
- Thénot S, Henriquet C, Rochefort H, Cavaillès V (1997). "Differential interaction of nuclear receptors with the putative human transcriptional coactivator hTIF1". J. Biol. Chem. 272 (18): 12062–8. doi:10.1074/jbc.272.18.12062. PMID 9115274.
- Lee WY, Noy N (2002). "Interactions of RXR with coactivators are differentially mediated by helix 11 of the receptor's ligand binding domain". Biochemistry. 41 (8): 2500–8. doi:10.1021/bi011764+. PMID 11851396.
- Monden T, Wondisford FE, Hollenberg AN (1997). "Isolation and characterization of a novel ligand-dependent thyroid hormone receptor-coactivating protein". J. Biol. Chem. 272 (47): 29834–41. doi:10.1074/jbc.272.47.29834. PMID 9368056.
- Jeyakumar M, Tanen MR, Bagchi MK (1997). "Analysis of the functional role of steroid receptor coactivator-1 in ligand-induced transactivation by thyroid hormone receptor". Mol. Endocrinol. 11 (6): 755–67. doi:10.1210/mend.11.6.0003. PMID 9171239.
- Baudino TA, Kraichely DM, Jefcoat SC, Winchester SK, Partridge NC, MacDonald PN (1998). "Isolation and characterization of a novel coactivator protein, NCoA-62, involved in vitamin D-mediated transcription". J. Biol. Chem. 273 (26): 16434–41. doi:10.1074/jbc.273.26.16434. PMID 9632709.
Further reading
- Szanto A, Narkar V, Shen Q, Uray IP, Davies PJ, Nagy L (December 2004). "Retinoid X receptors: X-ploring their (patho)physiological functions". Cell Death Differ. 11 (Suppl 2): S126–43. doi:10.1038/sj.cdd.4401533. PMID 15608692.
- Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, Thaller C (January 1992). "9-cis retinoic acid is a high affinity ligand for the retinoid X receptor". Cell. 68 (2): 397–406. doi:10.1016/0092-8674(92)90479-V. PMID 1310260. S2CID 39338234.
- Kliewer SA, Umesono K, Mangelsdorf DJ, Evans RM (January 1992). "Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling". Nature. 355 (6359): 446–9. Bibcode:1992Natur.355..446K. doi:10.1038/355446a0. PMC 6159885. PMID 1310351.
- Kliewer SA, Umesono K, Heyman RA, Mangelsdorf DJ, Dyck JA, Evans RM (February 1992). "Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling". Proc. Natl. Acad. Sci. U.S.A. 89 (4): 1448–52. Bibcode:1992PNAS...89.1448K. doi:10.1073/pnas.89.4.1448. PMC 48468. PMID 1311101.
- Bugge TH, Pohl J, Lonnoy O, Stunnenberg HG (April 1992). "RXR alpha, a promiscuous partner of retinoic acid and thyroid hormone receptors". EMBO J. 11 (4): 1409–18. doi:10.1002/j.1460-2075.1992.tb05186.x. PMC 556590. PMID 1314167.
- Berrodin TJ, Marks MS, Ozato K, Linney E, Lazar MA (September 1992). "Heterodimerization among thyroid hormone receptor, retinoic acid receptor, retinoid X receptor, chicken ovalbumin upstream promoter transcription factor, and an endogenous liver protein". Mol. Endocrinol. 6 (9): 1468–78. doi:10.1210/mend.6.9.1331778. PMID 1331778. S2CID 24291055.
- Mangelsdorf DJ, Umesono K, Kliewer SA, Borgmeyer U, Ong ES, Evans RM (August 1991). "A direct repeat in the cellular retinol-binding protein type II gene confers differential regulation by RXR and RAR". Cell. 66 (3): 555–61. doi:10.1016/0092-8674(81)90018-0. PMID 1651173. S2CID 13444623.
- Mangelsdorf DJ, Ong ES, Dyck JA, Evans RM (May 1990). "Nuclear receptor that identifies a novel retinoic acid response pathway". Nature. 345 (6272): 224–9. Bibcode:1990Natur.345..224M. doi:10.1038/345224a0. PMID 2159111. S2CID 4232833.
- Oñate SA, Tsai SY, Tsai MJ, O'Malley BW (1995). "Sequence and characterization of a coactivator for the steroid hormone receptor superfamily". Science. 270 (5240): 1354–7. Bibcode:1995Sci...270.1354O. doi:10.1126/science.270.5240.1354. PMID 7481822. S2CID 28749162.
- Chen JD, Evans RM (1995). "A transcriptional co-repressor that interacts with nuclear hormone receptors". Nature. 377 (6548): 454–7. Bibcode:1995Natur.377..454C. doi:10.1038/377454a0. PMID 7566127. S2CID 4361329.
- Schulman IG, Chakravarti D, Juguilon H, Romo A, Evans RM (August 1995). "Interactions between the retinoid X receptor and a conserved region of the TATA-binding protein mediate hormone-dependent transactivation". Proc. Natl. Acad. Sci. U.S.A. 92 (18): 8288–92. Bibcode:1995PNAS...92.8288S. doi:10.1073/pnas.92.18.8288. PMC 41142. PMID 7667283.
- Perlmann T, Jansson L (April 1995). "A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1". Genes Dev. 9 (7): 769–82. doi:10.1101/gad.9.7.769. PMID 7705655.
- Rastinejad F, Perlmann T, Evans RM, Sigler PB (1995). "Structural determinants of nuclear receptor assembly on DNA direct repeats". Nature. 375 (6528): 203–11. Bibcode:1995Natur.375..203R. doi:10.1038/375203a0. PMID 7746322. S2CID 4373097.
- Forman BM, Umesono K, Chen J, Evans RM (1995). "Unique response pathways are established by allosteric interactions among nuclear hormone receptors". Cell. 81 (4): 541–50. doi:10.1016/0092-8674(95)90075-6. PMID 7758108. S2CID 3203590.
- Seol W, Choi HS, Moore DD (1995). "Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors". Mol. Endocrinol. 9 (1): 72–85. doi:10.1210/mend.9.1.7760852. PMID 7760852.
- Bourguet W, Ruff M, Chambon P, Gronemeyer H, Moras D (June 1995). "Crystal structure of the ligand-binding domain of the human nuclear receptor RXR-alpha". Nature. 375 (6530): 377–82. Bibcode:1995Natur.375..377B. doi:10.1038/375377a0. PMID 7760929. S2CID 4371873.
- Lee JW, Choi HS, Gyuris J, Brent R, Moore DD (February 1995). "Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor". Mol. Endocrinol. 9 (2): 243–54. doi:10.1210/mend.9.2.7776974. PMID 7776974.
- Tontonoz P, Graves RA, Budavari AI, Erdjument-Bromage H, Lui M, Hu E, Tempst P, Spiegelman BM (December 1994). "Adipocyte-specific transcription factor ARF6 is a heterodimeric complex of two nuclear hormone receptors, PPAR gamma and RXR alpha". Nucleic Acids Res. 22 (25): 5628–34. doi:10.1093/nar/22.25.5628. PMC 310126. PMID 7838715.
- Lee JW, Ryan F, Swaffield JC, Johnston SA, Moore DD (March 1995). "Interaction of thyroid-hormone receptor with a conserved transcriptional mediator". Nature. 374 (6517): 91–4. Bibcode:1995Natur.374...91L. doi:10.1038/374091a0. PMID 7870181. S2CID 4324595.
- Lee MS, Sem DS, Kliewer SA, Provencal J, Evans RM, Wright PE (September 1994). "NMR assignments and secondary structure of the retinoid X receptor alpha DNA-binding domain. Evidence for the novel C-terminal helix". Eur. J. Biochem. 224 (2): 639–50. doi:10.1111/j.1432-1033.1994.00639.x. PMID 7925381.
External links
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
| PDB gallery | |
|---|---|
|
| Transcription factors and intracellular receptors | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
| see also transcription factor/coregulator deficiencies | |||||||||||||||||||||||||||||||
| Retinoid receptor modulators | |
|---|---|
| RARTooltip Retinoic acid receptor |
|
| RXRTooltip Retinoid X receptor | |
| |