 | |
| Clinical data | |
|---|---|
| Trade names | Arlidin |
| Other names | Nylidrin |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.531 |
| Chemical and physical data | |
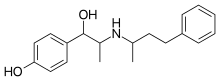
| Formula | C19H25NO2 |
| Molar mass | 299.414 g·mol |
| 3D model (JSmol) | |
SMILES
| |
Buphenine, also known as nylidrin and sold under the brand name Arlidin, is a β2 adrenoreceptor agonist that acts as a vasodilator.
It was developed as a chemical derivative of oxilofrine, and first reported in the literature in 1950.
See also
References
- Mittag TW, Tormay A, Messenger M, Podos SM (February 1985). "Ocular hypotension in the rabbit. Receptor mechanisms of pirbuterol and nylidrin". Invest Ophthalmol Vis Sci. 26 (2): 163–9. PMID 2857689. Archived from the original on 2013-04-15.
- Freedman L (1955). "Arlidin: a new vasodilative sympathomimetic drug". Angiology. 6 (1): 52–8. doi:10.1177/000331975500600106. PMID 14350296. S2CID 46317963.
- Külz F, Schneider M (1950). "Über neue gefäßerweiternde Sympathomimetika" [On new vasodilative sympathomimetics]. Klin Wochenschr (in German). 28 (31–32): 535–7. doi:10.1007/BF01481535. PMID 14775050.
| Tocolytics/labor repressants (G02CA) | |
|---|---|
| β2 adrenoreceptor agonists | |
| Oxytocin antagonists | |
| NSAIDs | |
| Calcium channel blockers | |
| Myosin inhibitors | |
| Peripheral vasodilators (C04) | |
|---|---|
| Phenylethanolamine derivatives | |
| Alpha blockers |
|
| Nicotinic acid and derivatives | |
| Purine derivatives | |
| Ergot alkaloids | |
| Other peripheral vasodilators | |
| Ionotropic glutamate receptor modulators | |
|---|---|
| AMPARTooltip α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
|
| KARTooltip Kainate receptor |
|
| NMDARTooltip N-Methyl-D-aspartate receptor |
|
This drug article relating to the cardiovascular system is a stub. You can help Misplaced Pages by expanding it. |
This drug article relating to the genito-urinary system is a stub. You can help Misplaced Pages by expanding it. |