| |
| Names | |
|---|---|
| IUPAC name Potassium peroxysulfate | |
| Other names
Caroat potassium monopersulfate MPS KMPS potassium caroate non-chlorine shock | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.030.158 |
| PubChem CID | |
| UNII |
|
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | KHSO5 |
| Molar mass | 152.2 g/mol (614.76 g/mol as triple salt) |
| Appearance | Off-white powder |
| Solubility in water | Decomposes |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Oxidant, corrosive |
| NFPA 704 (fire diamond) |
 |
| Safety data sheet (SDS) | Degussa Caroat MSDS |
| Related compounds | |
| Related compounds | Potassium persulfate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
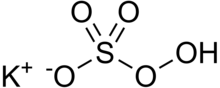
Potassium peroxymonosulfate is widely used as an oxidizing agent, for example, in pools and spas (usually referred to as monopersulfate or "MPS"). It is the potassium salt of peroxymonosulfuric acid. Potassium peroxymonosulfate per se is rarely encountered. It is often confused with the triple salt 2KHSO5·KHSO4·K2SO4, known as Oxone.
The standard electrode potential for potassium peroxymonosulfate is +1.81 V with a half reaction generating the hydrogen sulfate (pH = 0):
- HSO−5 + 2H + 2e → HSO−4 + H2O
Oxone
Potassium peroxymonosulfate per se is a relatively obscure salt, but its derivative called Oxone is of commercial value. Oxone refers to the triple salt 2KHSO5·KHSO4·K2SO4. As such about one third by weight is potassium peroxymonosulfate. Oxone has a longer shelf life than does potassium peroxymonosulfate. A white, water-soluble solid, Oxone loses <1% of its oxidizing power per month.
Oxone, which is commercially available, is produced from peroxysulfuric acid, which is generated in situ by combining oleum and hydrogen peroxide. Careful neutralization of this solution with potassium hydroxide allows the crystallization of the triple salt.
Uses
Cleaning
Oxone is used widely for cleaning. It whitens dentures, oxidizes organic contaminants in swimming pools,and cleans chips for the manufacture of microelectronics.
Organic oxidations
Oxone is a versatile oxidant in organic synthesis. It oxidizes aldehydes to carboxylic acids; in the presence of alcoholic solvents, the esters may be obtained. Internal alkenes may be cleaved to two carboxylic acids (see below), while terminal alkenes may be epoxidized. Sulfides give sulfones, tertiary amines give amine oxides, and phosphines give phosphine oxides.
Further illustrative of the oxidative power of this salt is the conversion of an acridine derivative to the corresponding acridine-N-oxide.

Oxone oxidizes sulfides to sulfoxides and then to sulfones.

Oxone converts ketones to dioxiranes, which are used for diverse oxidations in organic synthesis. The dominant reagent dimethyldioxirane (DMDO) forms upon treatment of acetone with oxone. Dioxiranes are versatile, especially for the epoxidation of olefins. Dioxiranes are also oxidize other unsaturated functionality, heteroatoms, and alkane C-H bonds.
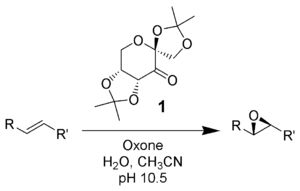
Other uses
Oxone has been investigated for the delignification of wood.
Ammonium, sodium, and potassium salts of H are used in the plastics industry as radical initiators for polymerization. They are also used as etchants, oxidative desizing agents for textile fabrics, and for decolorizing and deodorizing oils.
References
- Wu, Mingsong; Xu, Xinyang; Xu, Xun (November 2014). "Algicidal and Bactericidal Effect of Potassium Monopersulfate Compound on Eutrophic Water". Applied Mechanics and Materials. 707: 259. doi:10.4028/www.scientific.net/AMM.707.259. S2CID 98000605.
- "DuPont MSDS" (PDF). Archived from the original (PDF) on 2014-08-15. Retrieved 2012-01-26.
- Spiro, M. (1979). "The standard potential of the peroxosulphate/sulphate couple". Electrochimica Acta. 24 (3): 313–314. doi:10.1016/0013-4686(79)85051-3. ISSN 0013-4686.
- Crandall, Jack K.; Shi, Yian; Burke, Christopher P.; Buckley, Benjamin R. (2001). Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons, Ltd. doi:10.1002/047084289x.rp246.pub3. ISBN 978-0-470-84289-8.
- ^ Harald Jakob; Stefan Leininger; Thomas Lehmann; Sylvia Jacobi; Sven Gutewort. "Peroxo Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_177.pub2. ISBN 978-3527306732.
- Pool School. Trouble Free Pool. p. PT4. Retrieved November 30, 2018.
- Peroxy Compounds Human Health and Ecological Draft Risk Assessment DP 455445, 455446 (Report). United States Environmental Protection Agency. 2020-03-11. p. 9-10. Retrieved 2021-09-24.
- Wacławek, Stanisław; Lutze, Holger V.; Grübel, Klaudiusz; Padil, Vinod V.T.; Černík, Miroslav; Dionysiou, Dionysios. D. (2017-12-15). "Peroxy Compounds Human Health and Ecological Draft Risk Assessment DP 455445, 455446". Chemical Engineering Journal. 330: 44–62. doi:10.1016/j.cej.2017.07.132.
- Benjamin R. Travis; Meenakshi Sivakumar; G. Olatunji Hollist & Babak Borhan (2003). "Facile Oxidation of Aldehydes to Acids and Esters with Oxone". Organic Letters. 5 (7): 1031–4. doi:10.1021/ol0340078. PMID 12659566.
- Bell, Thomas W.; Cho, Young-Moon; Firestone, Albert; Healy, Karin; Liu, Jia; Ludwig, Richard; Rothenberger, Scott D. (1990). "9-n-Butyl-1,2,3,4,5,6,7,8-Octahydroacridin-4-ol". Organic Syntheses. 69: 226. doi:10.15227/orgsyn.069.0226.
- McCarthy, James R.; Matthews, Donald P.; P. Paolini, John (1995). "Reaction of Sulfoxides with Diethylaminosulfur Trifluoride". Organic Syntheses. 72: 209. doi:10.15227/orgsyn.072.0209.
- Adam, W.; Saha-Moller, C.; Zhao, C.-G. (2004). "Dioxirane Epoxidation of Alkenes". Org. React. 61: 219. doi:10.1002/0471264180.or061.02.
- Adam, W.; Zhao, C.-G.; Jakka, K. (2007). "Dioxirane Oxidations of Compounds other than Alkenes". Org. Reactions. 69: 1. doi:10.1002/0471264180.or069.01.
- Springer, E. L.; McSweeny, J. D. (1993). "Treatment of softwood kraft pulps with peroxymonosulfate before oxygen delignification". TAPPI Journal. 76 (8): 194–199. ISSN 0734-1415. Archived from the original on 2011-09-29. Retrieved 2011-05-14.
| Potassium compounds | |
|---|---|
| H, (pseudo)halogens | |
| chalcogens | |
| pnictogens | |
| B, C group | |
| transition metals | |
| organic | |
| Salts and covalent derivatives of the persulfate ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||