| |||
 1,4-dichlorobenzene crystallised on paper from DCM solution | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1,4-Dichlorobenzene | |||
| Other names
1,4-DCB para-Dichlorobenzene p-Dichlorobenzene p-DCB PDCB Paramoth Para crystals Paracide Dichlorocide | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 1680023 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.092 | ||
| EC Number |
| ||
| Gmelin Reference | 49722 | ||
| KEGG | |||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 3077 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C6H4Cl2 | ||
| Molar mass | 147.00 g·mol | ||
| Appearance | Colorless/white crystals | ||
| Odor | mothball-like | ||
| Density | 1.25 g/cm, solid | ||
| Melting point | 53.5 °C (128.3 °F; 326.6 K) | ||
| Boiling point | 174 °C (345 °F; 447 K) | ||
| Solubility in water | 10.5 mg/100 mL (20 °C) | ||
| Vapor pressure | 1.3 mmHg (20 °C) | ||
| Magnetic susceptibility (χ) | -82.93·10 cm/mol | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Suspected carcinogen | ||
| GHS labelling: | |||
| Pictograms |   
| ||
| Signal word | Warning | ||
| Hazard statements | H302, H315, H317, H319, H332, H335, H351, H410 | ||
| Precautionary statements | P201, P202, P261, P264, P270, P271, P272, P273, P280, P281, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P308+P313, P312, P321, P330, P332+P313, P333+P313, P337+P313, P362, P363, P391, P403+P233, P405, P501 | ||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | 66 °C (151 °F; 339 K) | ||
| Explosive limits | 2.5%-? | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) | 500 mg/kg (rat, oral) 2950 mg/kg (mouse, oral) 2512 mg/kg (rat, oral) 2830 mg/kg (rabbit, oral) | ||
| LDLo (lowest published) | 857 mg/kg (human, oral) 4000 mg/kg (rat, oral) 2800 mg/kg (guinea pig, oral) | ||
| NIOSH (US health exposure limits): | |||
| PEL (Permissible) | TWA 75 ppm (450 mg/m) | ||
| REL (Recommended) | Ca | ||
| IDLH (Immediate danger) | Ca | ||
| Related compounds | |||
| Related compounds | 1,2-Dichlorobenzene 1,3-Dichlorobenzene | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
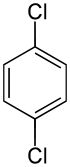
1,4-Dichlorobenzene (1,4-DCB, p-DCB, or para-dichlorobenzene, sometimes abbreviated as PDCB or para) is an aryl chloride and isomer of dichlorobenzene with the formula C6H4Cl2. This colorless solid has a strong odor. The molecule consists of a benzene ring with two chlorine atoms (replacing hydrogen atoms) on opposing sites of the ring.
It is used as a disinfectant, pesticide, and deodorant, most familiarly in mothballs in which it is a replacement for the more traditional naphthalene because of naphthalene's greater flammability (though both chemicals have the same NFPA 704 rating). It is also used as a precursor in the production of the chemically and thermally resistant polymer poly(p-phenylene sulfide).
Production
p-DCB is produced by chlorination of benzene using ferric chloride as a catalyst:
- C6H6 + 2 Cl2 → C6H4Cl2 + 2 HCl
The chief impurity is the 1,2 isomer. The compound can be purified by fractional crystallization, taking advantage of its relatively high melting point of 53.5 °C; the isomeric dichlorobenzenes and chlorobenzene melt well below room temperature.
Uses
Disinfectant, deodorant, and pesticide

p-DCB is used to control moths, molds, and mildew. It also finds use as a disinfectant in waste containers and restrooms and is the characteristic smell associated with urinal cakes. Its usefulness for these applications arises from p-DCB's low solubility in water and its relatively high volatility: it sublimes readily near room temperature.
Precursor to other chemicals
Nitration gives 1,4-dichloronitrobenzene, a precursor to commercial dyes and pigments. The chloride sites on p-DCB can be substituted with hydroxylamine and sulfide groups. In a growing application, p-DCB is the precursor to the high performance polymer poly(p-phenylene sulfide):
Environmental and health effects
p-DCB is poorly soluble in water and is not easily broken down by soil organisms. Like many hydrocarbons, p-DCB is lipophilic and will accumulate in fatty tissues if consumed by a person or animal.
The United States Department of Health and Human Services (DHHS) and the International Agency for Research on Cancer (IARC) have determined that p-DCB may reasonably be anticipated to be a carcinogen. This has been indicated by animal studies, although a full-scale human study has not been done.
The United States Environmental Protection Agency (EPA) has set a target maximum contaminant level of 75 micrograms of p-DCB per liter of drinking water (75 μg/L), but publishes no information on the cancer risk. p-DCB is also an EPA-registered pesticide. The United States Occupational Safety and Health Administration (OSHA) has set a maximum level of 75 parts of p-DCB per million parts air in the workplace (75 ppm) for an 8-hour day, 40-hour workweek.
A mechanism for the carcinogenic effects of mothballs and some types of air fresheners containing p-DCB has been identified in roundworms.
Due to its carcinogenic nature, use of paradichlorobenzene in the European Union is forbidden as an air freshener (since 2005) and in mothballs (since 2008).
Biodegradation
Rhodococcus phenolicus is a bacterium species able to degrade dichlorobenzene as its sole carbon source.
See also
References
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0190". National Institute for Occupational Safety and Health (NIOSH).
- ^ "p-Dichlorobenzene". National Institute for Occupational Safety and Health (NIOSH). 4 December 2014. Retrieved 6 March 2015.
- ^ Rossberg, M.; Lendle, W.; Pfleiderer, G.; Tögel, A.; Dreher, E. L.; Langer, E.; Rassaerts, H.; Kleinschmidt, P.; Strack, H.; Cook, R.; Beck, U.; Lipper, K.-A.; Torkelson, T.R.; Löser, E.; Beutel, K.K.; Mann, T. (2006). "Chlorinated Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_233.pub2. ISBN 978-3527306732.
- "National Pesticide Information Center – Mothballs Case Profile" (PDF). Archived from the original (PDF) on 22 June 2010. Retrieved 10 August 2009.
- K. Hunger. W. Herbst "Pigments, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012. doi:10.1002/14356007.a20_371
- Fahey, D. R.; Ash, C. E. (1991). "Mechanism of poly(p-phenylene sulfide) growth from p-dichlorobenzene and sodium sulfide". Macromolecules. 24 (15): 4242. Bibcode:1991MaMol..24.4242F. doi:10.1021/ma00015a003.
- Preamble to the IARC Monographs Archived 9 August 2016 at the Wayback Machine definition of "Group 2B: Possibly carcinogenic to humans", the International Agency for Research on Cancer classification of this chemical
- "ToxFAQs for Dichlorobenzenes". Toxic Substances Portal. Agency for Toxic Substances and Disease Registry. Retrieved 24 May 2013.
- "Consumer Factsheet on: PARA-DICHLOROBENZENE (p-DCB)". 28 November 2006. Archived from the original on 6 October 2009. Retrieved 10 August 2009.
- "1,4-Dichlorobenzene (para-Dichlorobenzene)". US Environmental Protection Agency. Archived from the original on 4 April 2016. Retrieved 24 March 2016.
- "Reregistration Eligibility Decision for Para-dichlorobenzene" (PDF). December 2008. Archived from the original (PDF) on 26 September 2009. Retrieved 10 August 2009.
- "Chemical Sampling – p-Diclorobenzine". United States Department of Labor. Occupational Safety & Health Administration. Archived from the original on 31 July 2017. Retrieved 23 March 2016.
- "Common Name: 1,4-DICHLOROBENZENE" (PDF). New Jersey Department of Health and Senior Services. December 2005. Retrieved 24 March 2016.
- Kokel, David (14 May 2006). "The nongenotoxic carcinogens naphthalene and para-dichlorobenzene suppress apoptosis in Caenorhabditis elegans". Nature Chemical Biology. 2 (6): 338–345. doi:10.1038/nchembio791. PMID 16699520. S2CID 18402091.
- Rehfuss, M.; Urban, J. (2005). "Rhodococcus phenolicus sp. nov., a novel bioprocessor isolated actinomycete with the ability to degrade chlorobenzene, dichlorobenzene and phenol as sole carbon sources". Systematic and Applied Microbiology. 28 (8): 695–701. doi:10.1016/j.syapm.2005.05.011. PMID 16261859.
External links
- International Chemical Safety Card 0037
- Mothball sniffing warning issued, BBC News, 27 July 2006
- NIOSH Pocket Guide to Chemical Hazards, Centers for Disease Control and Prevention