| |
| Names | |
|---|---|
| IUPAC name (5R)-4-thia-1-azabicycloheptan-7-one | |
| Other names 1-Aza-7-oxo-4-thiabicycloheptane | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 4374479 |
| ChEBI | |
| ChemSpider | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C5H7NOS |
| Molar mass | 129.18 g·mol |
| Related compounds | |
| Related compounds | clavam |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
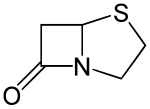
Penams are the primary skeleton structures that define the penicillin subclass of the broader β-lactam family of antibiotics and related compounds. They are bicyclic ring systems containing a β-lactam moiety fused with a five-member thiazolidine ring. Due to ring strain and limitations on amide resonance, the structure is unstable and highly susceptible to catalytic cleavage at the amide bond. Benzylpenicillin (penicillin G) is the natural product parent that contains the penam structure.
Structure
Penams have inflexible structures. The structure is locked in a puckered (i.e. bent) shape due to the pyramidal geometry of the bridgehead nitrogen. The pyramidalization (χ = 54°) and twist of the C-N bond (τ = 18°) is caused by the strain from the lone pair's exclusion from planarity with the cyclic rings and electrostatic repulsion effects. As a result, the distorted C-N bond causes misalignment the orbitals of the carbonyl carbon and the nitrogen lone pair that allow for resonance overlap. The amide C-N bond length is 1.406 Å and displays greater single bond character than in noncyclic tertiary amides. The C-O bond length is 1.205 Å which is shorter than C-O bonds in noncyclic tertiary amides.
Penams are strained due to the angle strain on the four-member β-lactam ring, whose internal bond angles are 90º. Consequently, penams are susceptible to acid- and base-catalyzed hydrolysis.
References
- ^ Novak, Igor; Chua, Pei Juan (2006-09-01). "Computational Study of Pharmacophores: β-Lactams". The Journal of Physical Chemistry A. 110 (35): 10521–10524. Bibcode:2006JPCA..11010521N. doi:10.1021/jp063162b. ISSN 1089-5639. PMID 16942059.
- Patrick, Graham (2017-03-23), "5. Pharmaceuticals and medicinal chemistry", Organic Chemistry: A Very Short Introduction, Oxford University Press, pp. 71–89, doi:10.1093/actrade/9780198759775.003.0005, ISBN 978-0-19-875977-5
- ^ Glover, Stephen A.; Rosser, Adam A. (2012-06-14). "Reliable Determination of Amidicity in Acyclic Amides and Lactams". The Journal of Organic Chemistry. 77 (13): 5492–5502. doi:10.1021/jo300347k. ISSN 0022-3263. PMID 22646836.
- ^ Hu, Feng; Lalancette, Roger; Szostak, Michal (2016-03-08). "Structural Characterization of N-Alkylated Twisted Amides: Consequences for Amide Bond Resonance and N−C Cleavage". Angewandte Chemie International Edition. 55 (16): 5062–5066. doi:10.1002/anie.201600919. ISSN 1433-7851. PMID 26953809.