
| |

| |
| Names | |
|---|---|
| IUPAC name Platinum tetrachloride | |
| Other names Platinum(IV) chloride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.300 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | PtCl4 |
| Molar mass | 336.89 g/mol |
| Appearance | brown-red powder |
| Density | 4.303 g/cm (anhydrous) 2.43 g/cm (pentahydrate) |
| Melting point | 370 °C (698 °F; 643 K) (decomposes) |
| Solubility in water | 58.7 g/100 mL (anhydrous) very soluble (pentahydrate) |
| Solubility | anhydrous soluble in acetone slightly soluble in ethanol insoluble in ether pentahydrate soluble in alcohol, ether |
| Magnetic susceptibility (χ) | −93.0·10 cm/mol |
| Structure | |
| Molecular shape | Square planar |
| Hazards | |
| GHS labelling: | |
| Pictograms |    
|
| Signal word | Danger |
| Hazard statements | H290, H301, H314, H317, H334 |
| Precautionary statements | P234, P260, P261, P264, P270, P272, P280, P285, P301+P310, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P304+P341, P305+P351+P338, P310, P321, P330, P333+P313, P342+P311, P363, P390, P404, P405, P501 |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 276 mg/kg (rat, oral) |
| Related compounds | |
| Other anions | Platinum(IV) bromide Platinum(IV) fluoride Platinum(IV) sulfide |
| Other cations | Iridium(IV) chloride |
| Related compounds | Platinum(II) chloride Platinum(VI) fluoride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Platinum(IV) chloride is the inorganic compound of platinum and chlorine with the empirical formula PtCl4. This brown solid features platinum in the 4+ oxidation state.
Structure
Typical of Pt(IV), the metal centers adopt an octahedral coordination geometry, {PtCl6}. This geometry is achieved by forming a polymer wherein half of the chloride ligands bridge between the platinum centers. Because of its polymeric structure, PtCl4 dissolves only upon breaking the chloride bridging ligands. Thus, addition of HCl give H2PtCl6. Lewis base adducts of Pt(IV) of the type cis-PtCl4L2 are known, but most are prepared by oxidation of the Pt(II) derivatives.
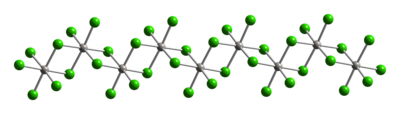
|
| Part of a (PtCl4)∞ chain from the crystal structure of platinum(IV) chloride |
Formation and reactions
PtCl4 is mainly encountered in the handling of chloroplatinic acid, obtained by dissolving of Pt metal in aqua regia. Heating H2PtCl6 to 220 °C gives impure PtCl4:
- H2PtCl6 → PtCl4 + 2 HCl
A purer product can be produced by heating under chlorine gas at 250 °C.
If excess acids are removed, PtCl4 crystallizes from aqueous solutions in large red crystals of pentahydrate PtCl4·5(H2O), which can be dehydrated by heating to about 300 °C in a current of dry chlorine. The pentahydrate is stable and is used as the commercial form of PtCl4.
Treatment of PtCl4 with aqueous base gives the ion. With methyl Grignard reagents followed by partial hydrolysis, PtCl4 converts to the cuboidal cluster 4. Upon heating PtCl4 evolves chlorine to give PtCl2:
- PtCl4 → PtCl2 + Cl2
The heavier halides, PtBr4 and PtI4, are also known.
References
- Cotton, S. A. Chemistry of Precious Metals, Chapman and Hall (London): 1997. ISBN 0-7514-0413-6.
- "Platinum tetrachloride". pubchem.ncbi.nlm.nih.gov. Retrieved 27 December 2021.
- M. F. Pilbrow (1972). "Crystal structure of platinum tetrachloride". Journal of the Chemical Society, Chemical Communications (5): 270–271. doi:10.1039/C39720000270.
- A. E. Schweizer; G. T. Kerr (1978). "Thermal decomposition of hexachloroplatinic acid". Inorganic Chemistry. 17 (8): 2326–2327. doi:10.1021/ic50186a067.
- Handbuch der präparativen anorganischen Chemie. 1 (3., umgearb. Aufl ed.). Stuttgart: Enke. 1975. p. 1709. ISBN 978-3-432-02328-1.
- George Samuel Newth (1920). A text-book of inorganic chemistry. Longmans, Green, and co. p. 694.
- Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
| Platinum compounds | |||
|---|---|---|---|
| Pt(−II) | |||
| Pt(0) | |||
| Pt(II) |
| ||
| Pt(IV) | |||
| Pt(V) | |||
| Pt(VI) | |||