| General | |
|---|---|
| Symbol | Po |
| Names | polonium-210, 210Po, Po-210, radium F |
| Protons (Z) | 84 |
| Neutrons (N) | 126 |
| Nuclide data | |
| Natural abundance | Trace |
| Half-life (t1/2) | 138.376±0.002 d |
| Isotope mass | 209.9828736 Da |
| Spin | 0 |
| Parent isotopes | Bi (β) |
| Decay products | Pb |
| Decay modes | |
| Decay mode | Decay energy (MeV) |
| Alpha decay | 5.40753 |
| Isotopes of polonium Complete table of nuclides | |
Polonium-210 (Po, Po-210, historically radium F) is an isotope of polonium. It undergoes alpha decay to stable Pb with a half-life of 138.376 days (about 4+1⁄2 months), the longest half-life of all naturally occurring polonium isotopes (Po). First identified in 1898, and also marking the discovery of the element polonium, Po is generated in the decay chain of uranium-238 and radium-226. Po is a prominent contaminant in the environment, mostly affecting seafood and tobacco. Its extreme toxicity is attributed to intense radioactivity, mostly due to alpha particles, which easily cause radiation damage, including cancer in surrounding tissue. The specific activity of
Po is 166 TBq/g, i.e., 1.66 × 10 Bq/g. At the same time, Po is not readily detected by common radiation detectors, because its gamma rays have a very low energy. Therefore,
Po can be considered as a quasi-pure alpha emitter.
History

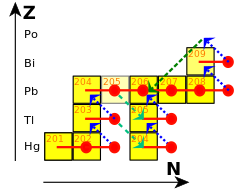
In 1898, Marie and Pierre Curie discovered a strongly radioactive substance in pitchblende and determined that it was a new element; it was one of the first radioactive elements discovered. Having identified it as such, they named the element polonium after Marie's home country, Poland. Willy Marckwald discovered a similar radioactive activity in 1902 and named it radio-tellurium, and at roughly the same time, Ernest Rutherford identified the same activity in his analysis of the uranium decay chain and named it radium F (originally radium E). By 1905, Rutherford concluded that all these observations were due to the same substance, Po. Further discoveries and the concept of isotopes, first proposed in 1913 by Frederick Soddy, firmly placed Po as the penultimate step in the uranium series.
In 1943, Po was studied as a possible neutron initiator in nuclear weapons, as part of the Dayton Project. In subsequent decades, concerns for the safety of workers handling Po led to extensive studies on its health effects.
In the 1950s, scientists of the United States Atomic Energy Commission at Mound Laboratories, Ohio explored the possibility of using Po in radioisotope thermoelectric generators (RTGs) as a heat source to power satellites. A 2.5-watt atomic battery using Po was developed by 1958. However, the isotope plutonium-238 was chosen instead, as it has a longer half-life of 87.7 years.
Polonium-210 was used to kill Russian dissident and ex-FSB officer Alexander V. Litvinenko in 2006, and was suspected as a possible cause of Yasser Arafat's death, following exhumation and analysis of his corpse in 2012–2013. The radioisotope may also have been used to kill Yuri Shchekochikhin, Lecha Islamov and Roman Tsepov.
Decay properties
Po is an alpha emitter that has a half-life of 138.376 days; it decays directly to stable Pb. The majority of the time, Po decays by emission of an alpha particle only, not by emission of an alpha particle and a gamma ray; about one in 100,000 decays results in the emission of a gamma ray.
This low gamma ray production rate makes it more difficult to find and identify this isotope. Rather than gamma ray spectroscopy, alpha spectroscopy is the best method of measuring this isotope.
Owing to its much shorter half-life, a milligram of Po emits as many alpha particles per second as 5 grams of Ra. A few curies of Po emit a blue glow caused by excitation of surrounding air.
Po occurs in minute amounts in nature, where it is the penultimate isotope in the uranium series decay chain. It is generated via beta decay from Pb and Bi.
The astrophysical s-process is terminated by the decay of Po, as the neutron flux is insufficient to lead to further neutron captures in the short lifetime of Po. Instead, Po alpha decays to Pb, which then captures more neutrons to become Po and repeats the cycle, thus consuming the remaining neutrons. This results in a buildup of lead and bismuth, and ensures that heavier elements such as thorium and uranium are only produced in the much faster r-process.
Production
Deliberate
Although Po occurs in trace amounts in nature, it is not abundant enough (0.1 ppb) for extraction from uranium ore to be feasible. Instead, most Po is produced synthetically, through neutron bombardment of Bi in a nuclear reactor. This process converts Bi to Bi, which beta decays to Po with a five-day half-life. Through this method, approximately 8 grams (0.28 oz) of Po are produced in Russia and shipped to the United States every month for commercial applications. By irradiating certain bismuth salts containing light element nuclei such as beryllium, a cascading (α,n) reaction can also be induced to produce Po in large quantities.
Byproduct
The production of polonium-210 is a downside to reactors cooled with lead-bismuth eutectic rather than pure lead. However, given the eutectic properties of this alloy, some proposed Generation IV reactor designs still rely on lead-bismuth.
Applications
A single gram of Po generates 140 watts of power. Because it emits many alpha particles, which are stopped within a very short distance in dense media and release their energy, Po has been used as a lightweight heat source to power thermoelectric cells in artificial satellites. A Po heat source was also in each of the Lunokhod rovers deployed on the surface of the Moon, to keep their internal components warm during the lunar nights. Some anti-static brushes, used for neutralizing static electricity on materials like photographic film, contain a few microcuries of Po as a source of charged particles. Po was also used in initiators for atomic bombs through the (α,n) reaction with beryllium. Small neutron sources reliant on the (α,n) reaction also usually use polonium as a convenient source of alpha particles due to its comparatively low gamma emissions (allowing easy shielding) and high specific activity.
Hazards
Main article: Polonium § Biology and toxicityPo is extremely toxic; it and other polonium isotopes are some of the most radiotoxic substances to humans. With one microgram of Po being more than enough to kill the average adult, it is 250,000 times more toxic than hydrogen cyanide by weight. One gram of Po would hypothetically be enough to kill 50 million people and sicken another 50 million. This is a consequence of its ionizing alpha radiation, as alpha particles are especially damaging to organic tissues inside the body. However, Po does not pose a radiation hazard when contained outside the body. The alpha particles it produces cannot penetrate the outer layer of dead skin cells.
The toxicity of Po stems entirely from its radioactivity. It is not chemically toxic in itself, but its solubility in aqueous solution as well as that of its salts poses a hazard because its spread throughout the body is facilitated in solution. Intake of Po occurs primarily through contaminated air, food, or water, as well as through open wounds. Once inside the body, Po concentrates in soft tissues (especially in the reticuloendothelial system) and the bloodstream. Its biological half-life is approximately 50 days.
In the environment, Po can accumulate in seafood. It has been detected in various organisms in the Baltic Sea, where it can propagate in, and thus contaminate, the food chain. Po is also known to contaminate vegetation, primarily originating from the decay of atmospheric radon-222 and absorption from soil.
In particular, Po attaches to, and concentrates in, tobacco leaves. Elevated concentrations of Po in tobacco were documented as early as 1964, and cigarette smokers were thus found to be exposed to considerably greater doses of radiation from Po and its parent Pb. Heavy smokers may be exposed to the same amount of radiation (estimates vary from 100 µSv to 160 mSv per year) as individuals in Poland were from Chernobyl fallout traveling from Ukraine. As a result, Po is most dangerous when inhaled from cigarette smoke.
References
- ^ Nuclear Data Center at KAERI; Table of Nuclides http://atom.kaeri.re.kr/nuchart/?zlv=1
- ^ Wang, M.; Audi, G.; Kondev, F. G.; Huang, W. J.; Naimi, S.; Xu, X. (2017). "The AME2016 atomic mass evaluation (II). Tables, graphs, and references" (PDF). Chinese Physics C. 41 (3): 030003-1–030003-442. doi:10.1088/1674-1137/41/3/030003.
- Thoennessen, M. (2016). The Discovery of Isotopes: A Complete Compilation. Springer. pp. 6–8. doi:10.1007/978-3-319-31763-2. ISBN 978-3-319-31761-8. LCCN 2016935977.
- ^ Roessler, G. (2007). "Why Po?" (PDF). Health Physics News. Vol. 35, no. 2. Health Physics Society. Archived (PDF) from the original on 2014-04-03. Retrieved 2019-06-20.
- Idaho National Laboratory (2015). "The Early Years: Space Nuclear Power Systems Take Flight" (PDF). Atomic power in space II: a history of space nuclear power and propulsion in the United States. pp. 2–5. OCLC 931595589.
- ^ McFee, R. B.; Leikin, J. B. (2009). "Death by polonium-210: lessons learned from the murder of former Soviet spy Alexander Litvinenko". Seminars in Diagnostic Pathology. 26 (1): 61–67. doi:10.1053/j.semdp.2008.12.003. PMID 19292030.
- Cowell, A. (November 24, 2006). "Radiation Poisoning Killed Ex-Russian Spy". The New York Times. Archived from the original on June 19, 2019. Retrieved June 19, 2019.
- "Arafat's death: what is Polonium-210?". Al Jazeera. July 10, 2012. Archived from the original on June 19, 2019. Retrieved June 19, 2019.
- Sweeney, J. (2022). Killer in the Kremlin. Penguin. p. 120. ISBN 9781804991206.
- "210Po A Decay". Korea Atomic Energy Research Institute. Archived from the original on February 24, 2015.
- C. R. Hammond. "The Elements" (PDF). Fermi National Accelerator Laboratory. pp. 4–22. Archived (PDF) from the original on 2008-06-26. Retrieved 2019-06-19.
- Burbidge, E. M.; Burbidge, G. R.; Fowler, W. A.; Hoyle, F. (1957). "Synthesis of the Elements in Stars". Reviews of Modern Physics. 29 (4): 547–650. Bibcode:1957RvMP...29..547B. doi:10.1103/RevModPhys.29.547.
- Lim, Solomon (2023). "Neutronic Chain Reactions for Polonium-210 Production" (PDF). SSRN. doi:10.2139/ssrn.4469519.
- "Polonium" (PDF). Argonne National Laboratory. Archived from the original (PDF) on 2012-03-10.
- A. Wilson, Solar System Log, (London: Jane's Publishing Company Ltd, 1987), p. 64.
- "Staticmaster Alpha Ionizing Brush". Company 7. Archived from the original on 2018-09-27. Retrieved 2019-06-19.
- Hoddeson, L.; Henriksen, P. W.; Meade, R. A. (2004). Critical Assembly: A Technical History of Los Alamos During the Oppenheimer Years, 1943–1945. Cambridge University Press. ISBN 978-0-521-54117-6.
- ^ Skwarzec, B.; Strumińska, D. I.; Boryło, A. (2006). "Radionuclides of iron (Fe), nickel (Ni), polonium (Po), uranium (U, U, U), and plutonium (Pu, Pu, Pu) in Poland and Baltic Sea environment" (PDF). Nukleonika. 51: S45–S51. Archived (PDF) from the original on 2019-06-19. Retrieved 2019-06-19.
- Ahmed, M. F.; Alam, L.; Mohamed, C. A. R.; Mokhtar, M. B.; Ta, G. C. (2018). "Health risk of polonium-210 ingestion via drinking water: An experience of Malaysia". International Journal of Environmental Research and Public Health. 15 (10): 2056–1–2056–19. doi:10.3390/ijerph15102056. PMC 6210456. PMID 30241360.
- "Radiation Studies: CDC - Radiation: Polonium-210 | CDC RSB". www.cdc.gov. 2019-02-11. Retrieved 2022-11-14.
- "Penetration Abilities of Different Types of Radiation". www.cdc.gov. Retrieved 2022-11-14.
- ^ Frequently asked questions about polonium-210 (PDF) (Report). Centers for Disease Control and Prevention. Archived (PDF) from the original on 7 June 2017. Retrieved 19 June 2019.
- Richter, F.; Wagmann, M.; Zehringer, M. (2012). "Polonium – on the Trace of a Powerful Alpha Nuclide in the Environment". CHIMIA International Journal for Chemistry. 66 (3): 131. doi:10.2533/chimia.2012.131. Archived from the original on 2019-02-17. Retrieved 2019-06-19.
- ^ Persson, B. R. R.; Holm, E. (2009). Polonium-210 and Lead-210 in the Terrestrial environment: A historical review. International Topical Conference on Po and Radioactive Pb Isotopes. Seville, Spain.
- "F. Typical Sources of Radiation Exposure". National Institute of Health. Archived from the original on 2013-06-13. Retrieved 2019-06-20.
- Radford, E. P.; Hunt, V. R. (1964). "Polonium-210: A Volatile Radioelement in Cigarettes". Science. 143 (3603): 247–249. Bibcode:1964Sci...143..247R. doi:10.1126/science.143.3603.247. JSTOR 1712451. PMID 14078362. S2CID 23455633.
