| |||
| Names | |||
|---|---|---|---|
| Other names
bromargyrite bromyrite silver(I) bromide | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.029.160 | ||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | AgBr | ||
| Molar mass | 187.77 g/mol | ||
| Appearance | Pale yellow solid photosensitive | ||
| Density | 6.473 g/cm, solid | ||
| Melting point | 432 °C (810 °F; 705 K) | ||
| Boiling point | 1,502 °C (2,736 °F; 1,775 K) (decomposes) | ||
| Solubility in water | 0.140 mg/L (20 °C) | ||
| Solubility product (Ksp) | 5.4 × 10 | ||
| Solubility | insoluble in alcohol, most acids sparingly soluble in ammonia soluble in alkali cyanide solutions | ||
| Band gap | 2.5 eV | ||
| Electron mobility | 4000 cm/(V·s) | ||
| Magnetic susceptibility (χ) | −59.7·10 cm/mol | ||
| Refractive index (nD) | 2.253 | ||
| Thermochemistry | |||
| Heat capacity (C) | 270 J/(kg·K) | ||
| Std molar entropy (S298) |
107 J·mol·K | ||
| Std enthalpy of formation (ΔfH298) |
−100 kJ·mol | ||
| Hazards | |||
| NFPA 704 (fire diamond) |
 | ||
| Related compounds | |||
| Other anions | Silver(I) fluoride Silver chloride Silver iodide | ||
| Other cations | Copper(I) bromide Mercury(I) bromide | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Silver bromide (AgBr), a soft, pale-yellow, water-insoluble salt well known (along with other silver halides) for its unusual sensitivity to light. This property has allowed silver halides to become the basis of modern photographic materials. AgBr is widely used in photographic films and is believed by some to have been used for making the Shroud of Turin. The salt can be found naturally as the mineral bromargyrite (bromyrite).
Preparation
Although the compound can be found in mineral form, AgBr is typically prepared by the reaction of silver nitrate with an alkali bromide, typically potassium bromide:
- AgNO3(aq) + KBr(aq) → AgBr(s)+ KNO3(aq)
Although less convenient, the salt can also be prepared directly from its elements.
Modern preparation of a simple, light-sensitive surface involves forming an emulsion of silver halide crystals in a gelatine, which is then coated onto a film or other support. The crystals are formed by precipitation in a controlled environment to produce small, uniform crystals (typically < 1 μm in diameter and containing ~10 Ag atoms) called grains.
Reactions
Silver bromide reacts readily with liquid ammonia to generate a variety of ammine complexes, like Ag(NH
3)
2Br and Ag(NH
3)
2Br
2. In general:
- AgBr + m NH3 + (n - 1) Br
→ Ag(NH
3)
mBr
n
Silver bromide reacts with triphenylphosphine to give a tris(triphenylphosphine) product:
Physical properties
Crystal structure
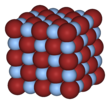
AgF, AgCl, and AgBr all have face-centered cubic (fcc) rock-salt (NaCl) lattice structure with the following lattice parameters:
The larger halide ions are arranged in a cubic close-packing, while the smaller silver ions fill the octahedral gaps between them, giving a 6-coordinate structure where a silver ion Ag is surrounded by 6 Br ions, and vice versa. The coordination geometry for AgBr in the NaCl structure is unexpected for Ag(I) which typically forms linear, trigonal (3-coordinated Ag) or tetrahedral (4-coordinated Ag) complexes.
Unlike the other silver halides, iodargyrite (AgI) contains a hexagonal zincite lattice structure.
Solubility
The silver halides have a wide range of solubilities. The solubility of AgF is about 6 × 10 times that of AgI. These differences are attributed to the relative solvation enthalpies of the halide ions; the enthalpy of solvation of fluoride is anomalously large.
| Compound | Solubility (g / 100 g H2O) |
| AgF | 172 |
| AgCl | 0.00019 |
| AgBr | 0.000014 |
| AgI | 0.000003 |
Photosensitivity
Although photographic processes have been in development since the mid-1800s, there were no suitable theoretical explanations until 1938 with the publication of a paper by R.W. Gurney and N.F. Mott. This paper triggered a large amount of research in fields of solid-state chemistry and physics, as well more specifically in silver halide photosensitivity phenomena.
Further research into this mechanism revealed that the photographic properties of silver halides (in particular AgBr) were a result of deviations from an ideal crystal structure. Factors such as crystal growth, impurities, and surface defects all affect concentrations of point ionic defects and electronic traps, which affect the sensitivity to light and allow for the formation of a latent image.
- Frenkel defects and quadropolar deformation
The major defect in silver halides is the Frenkel defect, where silver ions are located interstitially (Agi) in high concentration with their corresponding negatively charged silver-ion vacancies (Agv). What is unique about AgBr Frenkel pairs is that the interstitial Agi are exceptionally mobile, and that its concentration in the layer below the grain surface (called the space-charge layer) far exceeds that of the intrinsic bulk. The formation energy of the Frenkel pair is low at 1.16 eV, and the migration activation energy is unusually low at 0.05 eV (compare to NaCl: 2.18 eV for the formation of a Schottky pair and 0.75 eV for cationic migration). These low energies result in large defect concentrations, which can reach near 1% near the melting point.
The low activation energy in silver bromide can be attributed the silver ions' high quadrupolar polarizability; that is, it can easily deform from a sphere into an ellipsoid. This property, a result of the d electronic configuration of the silver ion, facilitates migration in both the silver ion and in silver-ion vacancies, thus giving the unusually low migration energy (for Agv: 0.29–0.33 eV, compared to 0.65 eV for NaCl).
Studies have demonstrated that the defect concentrations are strongly affected (up to several powers of 10) by crystal size. Most defects, such as interstitial silver ion concentration and surface kinks, are inversely proportional to crystal size, although vacancy defects are directly proportional. This phenomenon is attributed to changes in the surface chemistry equilibrium, and thus affects each defect concentration differently.
Impurity concentrations can be controlled by crystal growth or direct addition of impurities to the crystal solutions. Although impurities in the silver bromide lattice are necessary to encourage Frenkel defect formation, studies by Hamilton have shown that above a specific concentration of impurities, the numbers of defects of interstitial silver ions and positive kinks reduce sharply by several orders of magnitude. After this point, only silver-ion vacancy defects, which actually increase by several orders of magnitude, are prominent.
- Electron traps and hole traps
When light is incident on the silver halide grain surface, a photoelectron is generated when a halide loses its electron to the conduction band:
- X + hν → X + e
After the electron is released, it will combine with an interstitial Agi to create a silver metal atom Agi:
- e + Agi → Agi
Through the defects in the crystal, the electron is able to reduce its energy and become trapped in the atom. The extent of grain boundaries and defects in the crystal affect the lifetime of the photoelectron, where crystals with a large concentration of defects will trap an electron much faster than a purer crystal.
When a photoelectron is mobilized, a photohole h• is also formed, which also needs to be neutralized. The lifetime of a photohole, however, does not correlate with that of a photoelectron. This detail suggests a different trapping mechanism; Malinowski suggests that the hole traps may be related to defects as a result of impurities. Once trapped, the holes attract mobile, negatively charged defects in the lattice: the interstitial silver vacancy Agv:
- h• + Agv ⇌ h.Agv
The formation of the h.Agv lowers its energy sufficiently to stabilize the complex and reduce the probability of ejection of the hole back into the valance band (the equilibrium constant for hole-complex in the interior of the crystal is estimated at 10.
Additional investigations on electron- and hole-trapping demonstrated that impurities also can be a significant trapping system. Consequently, interstitial silver ions may not be reduced. Therefore, these traps are actually loss mechanisms, and are considered trapping inefficiencies. For example, atmospheric oxygen can interact with photoelectrons to form an O2 species, which can interact with a hole to reverse the complex and undergo recombination. Metal ion impurities such as copper(I), iron(II), and cadmium(II) have demonstrated hole-trapping in silver bromide.
- Crystal surface chemistry;
Once the hole-complexes are formed, they diffuse to the surface of the grain as a result of the formed concentration gradient. Studies demonstrated that the lifetimes of holes near the surface of the grain are much longer than those in the bulk, and that these holes are in equilibrium with adsorbed bromine. The net effect is an equilibrium push at the surface to form more holes. Therefore, as the hole-complexes reach the surface, they disassociate:
- h.Agv → h• + Agv → Br → FRACTION Br2
By this reaction equilibrium, the hole-complexes are constantly consumed at the surface, which acts as a sink, until removed from the crystal. This mechanism provides the counterpart to the reduction of the interstitial Agi to Agi, giving an overall equation of:
- AgBr → Ag + FRACTION Br2
- Latent image formation and photography
Now that some of the theory has been presented, the actual mechanism of the photographic process can be discussed. To summarize, as a photographic film is subjected to an image, photons incident on the grain produce electrons which interact to yield silver metal. More photons hitting a particular grain will produce a larger concentration of silver atoms, containing between 5 and 50 silver atoms (out of ~10 atoms), depending on the sensitivity of the emulsion. The film now has a concentration gradient of silver atom specks based upon varying intensity light across its area, producing an invisible "latent image".
While this process is occurring, bromine atoms are being produced at the surface of the crystal. To collect the bromine, a layer on top of the emulsion, called a sensitizer, acts as a bromine acceptor.
During film development the latent image is intensified by addition of a chemical, typically hydroquinone, that selectivity reduces those grains which contain atoms of silver. The process, which is sensitive to temperature and concentration, will completely reduce grains to silver metal, intensifying the latent image on the order of 10 to 10. This step demonstrates the advantage and superiority of silver halides over other systems: the latent image, which takes only milliseconds to form and is invisible, is sufficient to produce a full image from it.
After development, the film is "fixed", during which the remaining silver salts are removed to prevent further reduction, leaving the "negative" image on the film. The agent used is sodium thiosulfate, and reacts according to the following equation:
- AgX(s) + 2 Na2S2O3(aq) → Na3(aq) + NaX(aq)
An indefinite number of positive prints can be generated from the negative by passing light through it and undertaking the same steps outlined above.
Semiconductor properties
As silver bromide is heated within 100 °C of its melting point, an Arrhenius plot of the ionic conductivity shows the value increasing and "upward-turning". Other physical properties such as elastic moduli, specific heat, and the electronic energy gap also increase, suggesting the crystal is approaching instability. This behavior, typical of a semi-conductor, is attributed to a temperature-dependence of Frenkel defect formation, and, when normalized against the concentration of Frenkel defects, the Arrhenius plot linearizes.
See also
References
- ^ Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A23. ISBN 978-0-618-94690-7.
- ^ Greenwood, N.N., Earnshaw, A. (1984). Chemistry of the Elements. New York: Permagon Press. pp. 1185–87. ISBN 978-0-08-022057-4.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Hamilton, J.F. (1974). "Physical Properties of Silver Halide Microcrystals". Photographic Science and Engineering. 18 (5): 493–500.
- Leden, I., Persson, G.; Persson; Sjöberg; Dam; Sjöberg; Toft (1961). "The Solubility of Silver Chloride and Silver Bromide in Aqueous Ammonia and the Formation of Mixed Silver-Ammonia-Halide Complexes". Acta Chem. Scand. 15: 607–614. doi:10.3891/acta.chem.scand.15-0607.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Engelhardt, LM; Healy, PC; Patrick, VA; White, AH (1987). "Lewis-Base Adducts of Group-11 Metal(I) Compounds. XXX. 3:1 Complexes of Triphenylphosphine With Silver(I) Halides". Aust. J. Chem. 40 (11): 1873–1880. doi:10.1071/CH9871873.
- Glaus, S. & Calzaferri, G. (2003). "The band structures of the silver halides AgF, AgCl, and AgBr: A comparative study". Photochem. Photobiol. Sci. 2 (4): 398–401. Bibcode:2003PhPhS...2..398G. doi:10.1039/b211678b.
- Lide, David R. (ed). (2005)Handbook of Chemistry and Physics, 86th Edition, The Chemical Rubber Publishing Co., Cleveland.
- Gurney, R. W.; Mott, N. F. (1938). "The theory of the photolysis of silver bromide and the photographic latent image". Proc. Roy. Soc. A164 (917): 151–167. Bibcode:1938RSPSA.164..151G. doi:10.1098/rspa.1938.0011.
- ^ Slifkin, L. M. (1989). "The Physics of Lattice Defects in Silver Halides". Crystal Lattice Defects and Amorphous Materials. 18: 81–96.
- ^ Malinowski, J. (1968). "The Role of Holes in the Photographic Process". The Journal of Photographic Science. 16 (2): 57–62. doi:10.1080/00223638.1968.11737436.
| Silver compounds | |||
|---|---|---|---|
| Silver(0,I) | |||
| Silver(I) |
| ||
| Silver(II) | |||
| Silver(III) | |||
| Silver(I,III) | |||
| Bromine compounds | |
|---|---|
| Br(−I) | |
| Br(−I,I) | |
| Br(I) | |
| Br(II) | |
| Br(I,V) | |
| Br(III) | |
| Br(IV) | |
| Br(V) | |
| Br(VII) | |