| |
| Names | |
|---|---|
| Preferred IUPAC name 2-{oxy}-N,N,N-trimethylethan-1-aminium | |
| Other names Sinapoylcholine; Sinapic acid choline ester | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
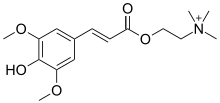
| Chemical formula | C16H24NO5 |
| Molar mass | 310.370 g·mol |
| Melting point | 178 °C (352 °F; 451 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Sinapine is an alkaloidal amine found in some seeds, particularly oil seeds of plants in the family Brassicaceae. It is the choline ester of sinapic acid.
Sinapine was discovered by Etienne Ossian Henry in 1825.
Occurrence
Sinapine typically occurs in the outer seed coat of oil crops and is plentiful in some types of press cake leftover after vegetable oil extraction. Typical oil seed cake residues high in sinapine include Brassica juncea (1.22% by mass), and rapeseed (0.39-1.06% by mass).
Isolation
The typical protocol for extracting Sinapine from seed cakes entails defatting the cake with hexane via a Soxhlet apparatus followed by extraction with 70% methanol held at 75 °C.
Metabolism
Sinapine esterase is an enzyme whose two substrates are sinapine and H2O and whose two products are sinapic acid and choline.
Sinapoylglucose—choline O-sinapoyltransferase is an enzyme whose two substrates are 1-O-sinapoyl-β-D-glucose and choline, whereas its two products are D-glucose and sinapine.
See also
- Phenolic content in wine
- Syringaldehyde
- Syringol
- Syringic acid
- Acetosyringone
- Sinapyl alcohol
- Sinapinic acid
- Sinapaldehyde
- Canolol
References
- Gmelin, R; Bredenberg JB, son (February 1966). "". Arzneimittel-Forschung (in German). 16 (2): 123–7. PMID 6014002.
- ^ Niciforovic, Neda; Abramovi, Helena (2014). "Sinapic Acid and Its Derivatives: Natural Sources and Bioactivity". Comprehensive Reviews in Food Science and Food Safety. 13 (1): 34–51. doi:10.1111/1541-4337.12041. PMID 33412688.
- Tzagoloff, A. (1963). "Metabolism of Sinapine in Mustard Plants. I. Degradation of Sinapine into Sinapic Acid & Choline". Plant Physiology. 38 (2): 202–206. doi:10.1104/pp.38.2.202. PMC 549906. PMID 16655775.
- Matthäus, B .; Zubr, J. (2000). "Variability of specific components in Camelina sativa oilseed cakes". Industrial Crops and Products. 12 (1): 9–18. doi:10.1016/S0926-6690(99)00040-0.
- Vuorela, Satu (2005). Analysis, isolation, and bioactivities of rapeseed phenolics (PDF). Helsinki, Finland: University of Helsinki. pp. 19–20. ISBN 9789521027215. Retrieved 14 June 2014.
- Vuorela, Satu (2005). Analysis, isolation, and bioactivities of rapeseed phenolics (PDF). Helsinki, Finland: University of Helsinki. pp. 19–20. ISBN 9789521027215. Retrieved 14 June 2014.