| |
| Names | |
|---|---|
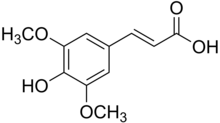
| Preferred IUPAC name (2E)-3-(4-Hydroxy-3,5-dimethoxyphenyl)prop-2-enoic acid | |
| Other names
Sinapinic acid Sinapic acid 3,5-Dimethoxy-4-hydroxycinnamic acid 4-Hydroxy-3,5-dimethoxycinnamic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C11H12O5 |
| Molar mass | 224.21 g/mol |
| Melting point | 203 to 205 °C (397 to 401 °F; 476 to 478 K) (decomposes) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Sinapinic acid, or sinapic acid (Sinapine - Origin: L. Sinapi, sinapis, mustard, Gr., cf. F. Sinapine.), is a small naturally occurring hydroxycinnamic acid. It is a member of the phenylpropanoid family. It is a commonly used matrix in MALDI mass spectrometry. It is a useful matrix for a wide variety of peptides and proteins. It serves well as a matrix for MALDI due to its ability to absorb laser radiation and to also donate protons (H) to the analyte of interest.
Sinapic acid can form dimers with itself (one structure) and ferulic acid (three different structures) in cereal cell walls and therefore may have a similar influence on cell-wall structure to that of the diferulic acids.
Sinapine is an alkaloidal amine found in black mustard seeds. It is considered a choline ester of sinapinic acid.
Natural occurrences
Sinapinic acid can be found in wine, vinegar, and black plums.
Metabolism
Sinapate 1-glucosyltransferase is an enzyme that uses UDP-glucose and sinapate to produce UDP and 1-sinapoyl-D-glucose.
Sinapoylglucose—malate O-sinapoyltransferase is an enzyme that uses 1-O-sinapoyl-beta-D-glucose and (S)-malate to produce D-glucose and sinapoyl-(S)-malate.
Related compounds
Canolol is a phenolic compound found in crude canola oil. It is produced by decarboxylation of sinapic acid during canola seed roasting.
See also
- Phenolic content in wine
- Syringaldehyde
- Syringol
- Syringic acid
- Acetosyringone
- Sinapyl alcohol
- Sinapaldehyde
- Sinapine
- Canolol
References
- Beavis RC, Chait BT (1989). "Matrix-assisted laser-desorption mass spectrometry using 355 nm radiation". Rapid Commun. Mass Spectrom. 3 (12): 436–9. Bibcode:1989RCMS....3..436B. doi:10.1002/rcm.1290031208. PMID 2520224.
- Beavis RC, Chait BT (1989). "Cinnamic acid derivatives as matrices for ultraviolet laser desorption mass spectrometry of proteins". Rapid Commun. Mass Spectrom. 3 (12): 432–5. Bibcode:1989RCMS....3..432B. doi:10.1002/rcm.1290031207. PMID 2520223.
- Bunzel M, Ralph J, Kim H, Lu F, Ralph SA, Marita JM, Hatfield RD, Steinhart H (2003). "Sinapate dehydrodimers and sinapate-ferulate heterodimers in cereal dietary fibre". J. Agric. Food Chem. 51 (5): 1427–1434. doi:10.1021/jf020910v. PMID 12590493.
- Tzagoloff A (1963). "Metabolism of Sinapine in Mustard Plants. I. Degradation of Sinapine into Sinapic Acid & Choline". Plant Physiology. 38 (2): 202–206. doi:10.1104/pp.38.2.202. PMC 549906. PMID 16655775.
- Comparison of Phenolic Acids and Flavan-3-ols During Wine Fermentation of Grapes with Different Harvest Times. Rong-Rong Tian, Qiu-Hong Pan, Ji-Cheng Zhan, Jing-Ming Li, Si-Bao Wan, Qing-Hua Zhang and Wei-Dong Huang, Molecules, 2009, 14, pages 827-838, doi:10.3390/molecules14020827
- Gávez MC, Barroso CG, Péez-Bustamante JA (1994). "Analysis of polyphenolic compounds of different vinegar samples". Zeitschrift für Lebensmittel-Untersuchung und -Forschung. 199: 29–31. doi:10.1007/BF01192948. S2CID 91784893.
- Jawad M, Ali M, Qasim S, Akbar A, Khan NA, Sadiq MB (2022-08-02). "Determination of Phenolic Compounds and Bioactive Potential of Plum (Prunus salicina) Peel Extract Obtained by Ultrasound-Assisted Extraction". BioMed Research International. 2022: 7787958. doi:10.1155/2022/7787958. ISSN 2314-6133. PMC 9433295. PMID 36060132.
- Antioxidant canolol production from a renewable feedstock via an engineered decarboxylase. Krista L. Morley, Stephan Grosse, Hannes Leischa and Peter C. K. Lau, Green Chem., 2013,n15, pages 3312-3317, doi:10.1039/C3GC40748A
| Types of hydroxycinnamic acids | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aglycones |
| ||||||||||||||||
| Esters |
| ||||||||||||||||
| Oligomeric forms |
| ||||||||||||||||
| Conjugates with coenzyme A (CoA) | |||||||||||||||||