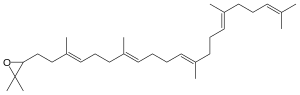
| |
| Names | |
|---|---|
| Preferred IUPAC name 2,2-Dimethyl-3-oxirane | |
| Other names
Squalene oxide 2,3-Squalene oxide Squalene epoxide Squalene-2,3-epoxide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| MeSH | 2,3-oxidosqualene |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C30H50O |
| Molar mass | 426.717 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
(S)-2,3-Oxidosqualene ((S)-2,3-epoxysqualene) is an intermediate in the synthesis of the cell membrane sterol precursors lanosterol and cycloartenol, as well as saponins. It is formed when squalene is oxidized by the enzyme squalene monooxygenase. 2,3-Oxidosqualene is the substrate of various oxidosqualene cyclases, including lanosterol synthase, which produces lanosterol, a precursor to cholesterol.
The stereoisomer (R)-2,3-oxidosqualene is an inhibitor of lanosterol synthase.
References
- Abe I. (2007). "Enzymatic synthesis of cyclic triterpenes". Natural Product Reports. 24 (6): 1311–31. doi:10.1039/b616857b. PMID 18033581.
External links
| Cholesterol and steroid metabolic intermediates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mevalonate pathway |
| ||||||||||
| Non-mevalonate pathway | |||||||||||
| To Cholesterol | |||||||||||
| From Cholesterol to Steroid hormones |
| ||||||||||
| Nonhuman |
| ||||||||||