| |
| Names | |
|---|---|
| IUPAC name 5ξ-Stigmastane | |
| Systematic IUPAC name (1R,3aS,3bR,5aΞ,9aS,9bS,11aR)-1--9a,11a-dimethylhexadecahydro-1H-cyclopentaphenanthrene | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| Beilstein Reference | 8170826 |
| ChEBI | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C29H52 |
| Molar mass | 400.735 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
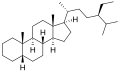
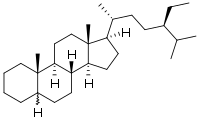
Stigmastane or 24R-ethylcholestane is a tetracyclic triterpene, along with cholestane and ergostane, this sterane is used as a biomarker for early eukaryotes.
See also
- Stigmastanol (Stigmastan-3β-ol)
- β-Sitosterol (Stigmast-5-en-3β-ol)
- Stigmasterol (Stigmast-5,22-dien-3β-ol)
References
- International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 1531. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- Brocks, Jochen J.; Jarrett, Amber J. M.; Sirantoine, Eva; Hallmann, Christian; Hoshino, Yosuke; Liyanage, Tharika (2017). "The rise of algae in Cryogenian oceans and the emergence of animals". Nature. 548 (7669): 578–581. Bibcode:2017Natur.548..578B. doi:10.1038/nature23457. PMID 28813409. S2CID 205258987.