| |||
| Names | |||
|---|---|---|---|
| Other names Pentafluorochlorosulfanyl | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.034.014 | ||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | SF5Cl | ||
| Molar mass | 162.510 g/mol | ||
| Appearance | Colorless gas | ||
| Density | 6.642 g/dm | ||
| Melting point | −64 °C (−83 °F; 209 K) | ||
| Boiling point | −19 °C (−2 °F; 254 K) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Toxic | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
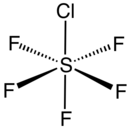
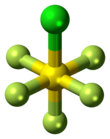
Sulfur chloride pentafluoride is an inorganic compound with the formula SF5Cl. It exists as a colorless gas at room temperature and is highly toxic, like most inorganic compounds containing the pentafluorosulfide (–SF5) functional group. The compound adopts an octahedral geometry with C
4v symmetry. Sulfur chloride pentafluoride is the only commercially available reagent for adding the –SF5 group to organic compounds.
Reactivity
SF5Cl is highly reactive and toxic. In contrast, sulfur hexafluoride (SF6) is inert and nontoxic despite having a closely related chemical formula. This difference highlights the lability of the S–Cl bond in SF5Cl.
Under free-radical conditions, SF5Cl adds across double bonds. The following reaction occurs with propene:
- CH
3CH=CH
2 + SF
5Cl → CH3CHClCH2SF5
The addition reaction is catalyzed by (CH3CH2)3B at around −30 °C. SF5Br is used similarly.
SF5Cl is also a precursor to O(SF5)2 and F2NSF5 (from tetrafluorohydrazine).
Synthesis
Sulfur chloropentafluoride can be synthesized by several routes, starting from two lower sulfur fluorides, sulfur tetrafluoride and disulfur decafluoride:
- SF4 + Cl2 + CsF → SF5Cl + CsCl
- ClF + SF4 → SF5Cl
- S2F10 + Cl2 → 2 SF5Cl
The corresponding SF
5Br is prepared similarly from in-situ generated bromine monofluoride.
References
- ^ Nyman, F., Roberts, H. L., Seaton, T. "Sulfur Chloride Pentafluoride" Inorganic Syntheses, 1966, Volume 8, p. 160. doi:10.1002/9780470132395.ch42
- ^ Dolbier, William R.; et al. (2006). "A convenient and efficient method for incorporation of pentafluorosulfanyl (SF5) substituents into aliphatic compounds". Journal of Fluorine Chemistry. 127 (10): 1302–10. doi:10.1016/j.jfluchem.2006.05.003.
- Savoie, Paul R.; Welch, John T. (2015). "Preparation and Utility of Organic Pentafluorosulfanyl-Containing Compounds". Chemical Reviews. 115 (2): 1130–1190. doi:10.1021/cr500336u. PMID 25341449.
- Winter, Rolf; Terjeson, Robin J.; Gard, Gary L. (1998). "An Improved and Facile Preparation of SF5Br". Journal of Fluorine Chemistry. 89: 105–106. doi:10.1016/S0022-1139(98)00094-3.