| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.033.228 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
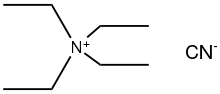
| Chemical formula | C9H20N2 |
| Molar mass | 156.273 g·mol |
| Appearance | white solid |
| Melting point | 254 °C (489 °F; 527 K) |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H300, H310, H330, H410 |
| Precautionary statements | P260, P262, P264, P270, P271, P273, P280, P284, P301+P310, P302+P350, P304+P340, P310, P320, P321, P322, P330, P361, P363, P391, P403+P233, P405, P501 |
| Related compounds | |
| Other anions | Tetraethylammonium chloride Tetraethylammonium bromide Tetraethylammonium iodide |
| Other cations | Tetramethylammonium cyanide Ammonium cyanide Guanidinium cyanide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Tetraethylammonium cyanide is the organic compound with the formula (C2H5)4NCN. It is a "quat salt" of cyanide. It is a colorless, deliquescent solid that is soluble in polar organic media. It is used in the synthesis of cyanometallates.
Tetraethylammonium cyanide is prepared by ion exchange from tetraethylammonium bromide. The corresponding tetraphenylarsonium salt is prepared similarly.
Safety
The salt is highly toxic as it contains cyanide ions.
See also
References
- Entley, William R.; Treadway, Christopher R.; Wilson, Scott R.; Girolami, Gregory S. (1997). "The Hexacyanotitanate Ion: Synthesis and Crystal Structure of [NEt4]3[Ti(CN)6]·4MeCN". Journal of the American Chemical Society. 119 (27): 6251–6258. doi:10.1021/ja962773m.
- Dieck, R. L.; Peterson, E. J.; Galliart, A.; Brown, T. M.; Moeller, T. (1976). "Tetraethylammonium, Tetraphenylarsonium, and Ammonium Cyanates and Cyanides". Inorganic Syntheses. Vol. 16. pp. 131–137. doi:10.1002/9780470132470.ch36. ISBN 9780470132470.