| thyrotropin-releasing hormone | |||||||
|---|---|---|---|---|---|---|---|
 Structural formula of TRH Structural formula of TRH | |||||||
| Identifiers | |||||||
| Symbol | TRH | ||||||
| NCBI gene | 7200 | ||||||
| HGNC | 12298 | ||||||
| OMIM | 275120 | ||||||
| RefSeq | NM_007117 | ||||||
| UniProt | P20396 | ||||||
| Other data | |||||||
| Locus | Chr. 3 q13.3-q21 | ||||||
| |||||||
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.934 |
| Chemical and physical data | |
| Formula | C16H22N6O4 |
| Molar mass | 362.390 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Thyrotropin-releasing hormone (TRH) is a hypophysiotropic hormone produced by neurons in the hypothalamus that stimulates the release of thyroid-stimulating hormone (TSH) and prolactin from the anterior pituitary.
TRH has been used clinically for the treatment of spinocerebellar degeneration and disturbance of consciousness in humans. Its pharmaceutical form is called protirelin (INN) (/proʊˈtaɪrɪlɪn/).
Synthesis and release
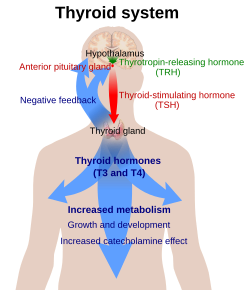
TRH is synthesized within parvocellular neurons of the paraventricular nucleus of the hypothalamus. It is translated as a 242-amino acid precursor polypeptide that contains 6 copies of the sequence -Gln-His-Pro-Gly-, with both ends of the sequence flanked by Lys-Arg or Arg-Arg sequences.
To produce the mature form, a series of enzymes are required. First, a protease cleaves to the C-terminal side of the flanking Lys-Arg or Arg-Arg. Second, a carboxypeptidase removes the Lys/Arg residues leaving Gly as the C-terminal residue. Then, this Gly is converted into an amide residue by a series of enzymes collectively known as peptidylglycine-alpha-amidating monooxygenase. Concurrently with these processing steps, the N-terminal Gln (glutamine) is converted into pyroglutamate (a cyclic residue). These multiple steps produce 6 copies of the mature TRH molecule per precursor molecule for human TRH (5 for mouse TRH).
TRH synthesizing neurons of the paraventricular nucleus project to the medial portion of the external layer of the median eminence. Following secretion at the median eminence, TRH travels to the anterior pituitary via the hypophyseal portal system where it binds to the TRH receptor stimulating the release of thyroid-stimulating hormone from thyrotropes and prolactin from lactotropes. The half-life of TRH in the blood is approximately 6 minutes.
Structure
TRH is a tripeptide, with an amino acid sequence of pyroglutamyl-histidyl-proline amide.
History
The structure of TRH was first determined, and the hormone synthesized, by Roger Guillemin and Andrew V. Schally in 1969. Both parties insisted their labs determined the sequence first: Schally first suggested the possibility in 1966, but abandoned it after Guillemin proposed TRH was not actually a peptide. Guillemin's chemist began concurring with these results in 1969, as NIH threatened to cut off funding for the project, leading both parties to return to work on synthesis.
Schally and Guillemin shared the 1977 Nobel Prize in Medicine "for their discoveries concerning the peptide hormone production of the brain." News accounts of their work often focused on their "fierce competition" and use of a very large amount of sheep and pig brains to locate the hormone.
Clinical significance
TRH is used clinically by intravenous injection (brand name Relefact TRH) to test the response of the anterior pituitary gland; this procedure is known as a TRH test. This is done as diagnostic test of thyroid disorders such as secondary hypothyroidism and in acromegaly.
TRH has anti-depressant and anti-suicidal properties, and in 2012 the U.S. Army awarded a research grant to develop a TRH nasal spray in order to prevent suicide amongst its ranks. The antidepressant properties of TRH are present when TRH is administered intrathecally, or administration into the spine, and the effects are short-lived. Some researchers are testing a prodrug approach to administer TRH orally and have TRH reach the brain without being degraded in the stomach or blood.
TRH has been shown in mice to be an anti-aging agent with a broad spectrum of activities that, because of their actions, suggest that TRH has a fundamental role in the regulation of metabolic and hormonal functions.
Side effects
Side effects after intravenous TRH administration are minimal. Nausea, flushing, urinary urgency, and mild rise in blood pressure have been reported. After intrathecal administration, shaking, sweating, shivering, restlessness, and mild rise in blood pressure were observed.
| Thyrotropin-releasing hormone (TRH) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | TRH | ||||||||
| Pfam | PF05438 | ||||||||
| InterPro | IPR008857 | ||||||||
| |||||||||
See also
- Thyrotropin-releasing hormone receptor
- Thyroid-stimulating hormone
- Hypothalamic–pituitary–thyroid axis
- Hypothalamic–pituitary–prolactin axis
References
- Urayama A, Yamada S, Kimura R, Zhang J, Watanabe Y (December 2002). "Neuroprotective effect and brain receptor binding of taltirelin, a novel thyrotropin-releasing hormone (TRH) analogue, in transient forebrain ischemia of C57BL/6J mice". Life Sciences. 72 (4–5): 601–607. doi:10.1016/S0024-3205(02)02268-3. PMID 12467901.
- Taylor T, Wondisford FE, Blaine T, Weintraub BD (January 1990). "The paraventricular nucleus of the hypothalamus has a major role in thyroid hormone feedback regulation of thyrotropin synthesis and secretion". Endocrinology. 126 (1): 317–324. doi:10.1210/endo-126-1-317. PMID 2104587.
- Bowen R (1998-09-20). "Thyroid-Stimulating Hormone". Pathophysiology of the Endocrine System. Colorado State University. Retrieved 2009-03-04.
- Boler J, Enzmann F, Folkers K, Bowers CY, Schally AV (November 1969). "The identity of chemical and hormonal properties of the thyrotropin releasing hormone and pyroglutamyl-histidyl-proline amide". Biochemical and Biophysical Research Communications. 37 (4): 705–710. doi:10.1016/0006-291X(69)90868-7. PMID 4982117.
- Burgus R, Dunn TF, Desiderio D, Guillemin R (November 1969). "". Comptes Rendus de l'Académie des Sciences, Série D (in French). 269 (19): 1870–1873. PMID 4983502.
- ^ Woolgar S, Latour B (1979). "Chapter 3: The Case of TRF(H)". Laboratory life: the social construction of scientific facts. Thousand Oaks: Sage Publications. ISBN 0-8039-0993-4.
- "The Nobel Prize in Physiology or Medicine 1977". NobelPrize.org. Retrieved 2009-03-04.
- ^ Marangell LB, George MS, Callahan AM, Ketter TA, Pazzaglia PJ, L'Herrou TA, et al. (March 1997). "Effects of intrathecal thyrotropin-releasing hormone (protirelin) in refractory depressed patients". Archives of General Psychiatry. 54 (3): 214–222. doi:10.1001/archpsyc.1997.01830150034007. PMID 9075462.
- "Scientist developing anti-suicide nasal spray". ArmyTimes.com. 25 July 2012. Retrieved 2012-07-05.
- "Army anti-suicide initiative brings $3 million to IU School of Medicine scientist's research". Indiana University School of Medicine. July 24, 2012.
- Prokai-Tatrai K, De La Cruz DL, Nguyen V, Ross BP, Toth I, Prokai L (July 2019). "Brain Delivery of Thyrotropin-Releasing Hormone via a Novel Prodrug Approach". Pharmaceutics. 11 (7): 349. doi:10.3390/pharmaceutics11070349. PMC 6680701. PMID 31323784.
- Pierpaoli W (February 2013). "Aging-reversing properties of thyrotropin-releasing hormone". Current Aging Science. 6 (1): 92–8. doi:10.2174/1874609811306010012. PMID 23895526.
- Prange AJ, Lara PP, Wilson IC, Alltop LB, Breese GR (November 1972). "Effects of thyrotropin-releasing hormone in depression". Lancet. 2 (7785): 999–1002. doi:10.1016/S0140-6736(72)92407-5. PMID 4116985. S2CID 40592228.
- Borowski GD, Garofano CD, Rose LI, Levy RA (January 1984). "Blood pressure response to thyrotropin-releasing hormone in euthyroid subjects". The Journal of Clinical Endocrinology and Metabolism. 58 (1): 197–200. doi:10.1210/jcem-58-1-197. PMID 6417153.
External links
 Media related to Thyrotropin-releasing hormone at Wikimedia Commons
Media related to Thyrotropin-releasing hormone at Wikimedia Commons
| Peptides: neuropeptides | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hormones | see hormones | ||||||||||
| Opioid peptides |
| ||||||||||
| Other neuropeptides |
| ||||||||||
| Galactagogues | |||||
|---|---|---|---|---|---|
| Prolactin receptor agonists | |||||
| Prolactin releasers |
| ||||
| Others |
| ||||