In organic chemistry, two molecules are valence isomers when they are constitutional isomers that can interconvert through pericyclic reactions.
Benzene
There are many valence isomers one can draw for the C6H6 formula benzene. Some were originally proposed for benzene itself before the actual structure of benzene was known. Others were later synthesized in lab. Some have been observed to isomerize to benzene, whereas others tend to undergo other reactions instead, or isomerize by ways other than pericyclic reactions.
- Some known valence isomers of benzene
-
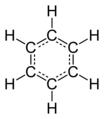 Benzene
Benzene
-
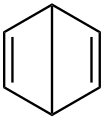 Dewar benzene
Dewar benzene
-
 Prismane
Prismane
-
 Benzvalene
Benzvalene
-
 Bicyclopropenyl
Bicyclopropenyl
Cyclooctatetraene
The valence isomers are not restricted to isomers of benzene. Valence isomers are also seen in the series (CH)8. Due to the larger number of units, the number of possible valence isomers is also greater and at least 21:
- Valence isomers of cyclooctatetraene
-
 Cyclooctatetraene (COT)
Cyclooctatetraene (COT)
-
 Barrelene
Barrelene
-
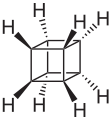 Cubane
Cubane
-
 Cuneane
Cuneane
-
 Semibullvalene
Semibullvalene
-
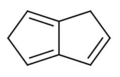 1,5-dihydropentalene
1,5-dihydropentalene
-
 2a,2b,4a,4b-Tetrahydrocyclopropapentalene
2a,2b,4a,4b-Tetrahydrocyclopropapentalene
-
 Bicycloocta-2,4,7-triene. Tautomer with COT by thermal 6e process or photochemical 4e process
Bicycloocta-2,4,7-triene. Tautomer with COT by thermal 6e process or photochemical 4e process
-
 Tricycloocta-3,8-diene. Isomerises to semibullvalene at room temperature, stable at −60 °C
Tricycloocta-3,8-diene. Isomerises to semibullvalene at room temperature, stable at −60 °C
-
 Tricycloocta-3,7-diene. The dimer of cyclobutadiene occurs as a cis isomer and a trans isomer. Both isomers convert to COT (symmetry forbidden hence stable) with a half-life of 20 minutes at 140 °C
Tricycloocta-3,7-diene. The dimer of cyclobutadiene occurs as a cis isomer and a trans isomer. Both isomers convert to COT (symmetry forbidden hence stable) with a half-life of 20 minutes at 140 °C
-
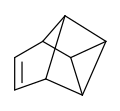 Tetracyclo octa-7-ene is only known as its 4-carbomethoxy derivative.
Tetracyclo octa-7-ene is only known as its 4-carbomethoxy derivative.
-
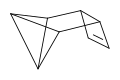 Tetracyclo octa-7-ene has been prepared from benzvalene and isomerises to COT
Tetracyclo octa-7-ene has been prepared from benzvalene and isomerises to COT
-
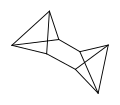 Pentacyclooctane (octabisvalene) is the third saturated valence isomer. The (Z)-3,7-phenylsulfonyl derivative is stable up to 200 °C.
Pentacyclooctane (octabisvalene) is the third saturated valence isomer. The (Z)-3,7-phenylsulfonyl derivative is stable up to 200 °C.
-
 Tricycloocta-3,5-diene (octavalene) was reported synthesised from homobenzvalene and converts to COT at 50 °C
Tricycloocta-3,5-diene (octavalene) was reported synthesised from homobenzvalene and converts to COT at 50 °C
Naphthalene and azulene
Perhaps no pair of valence isomers differ more strongly in appearance than colourless naphthalene and the intensely violet azulene.
- The valence isomers of naphthalene
-
 Naphthalene
Naphthalene
-
 Azulene
Azulene
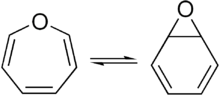
Benzene oxide and oxepin
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (1994) "Valence isomer". doi:10.1351/goldbook.V06590
- Rearrangements and interconversions of compounds of the formula (CH)n Lawrence T. Scott, Maitland. Jones Chem. Rev., 1972, 72 (2), pp 181–202 doi:10.1021/cr60276a004
- Huisgen, R.; Mietzsch, F. (1964). "The Valence Tautomerism of Cyclooctatetraene". Angewandte Chemie International Edition in English. 3 (2): 83. doi:10.1002/anie.196400831.
- Bicycloocta-2,4,7-triene Emanuel Vogel, H. Kiefer, W. R. Roth Volume 3, Issue 6, pages 442–443, June 1964 doi:10.1002/anie.196404422
- Huisgen, Rolf.; Konz, Will E.; Gream, George E. (1970). "Evidence for different valence tautomers of bromocyclooctatetraene". Journal of the American Chemical Society. 92 (13): 4105. doi:10.1021/ja00716a048.
- Meinwald, Jerrold; Tsuruta, Haruki (1969). "Tricycloocta-3,7-diene". Journal of the American Chemical Society. 91 (21): 5877. doi:10.1021/ja01049a034.
- Meinwald, Jerrold; Schmidt, Douglass (1969). "Semibullvalene from tricyclooctane". Journal of the American Chemical Society. 91 (21): 5877. doi:10.1021/ja01049a033.
- Zimmerman, Howard Elliot; Robbins, Jeffrey D.; Schantl, Joachim (1969). "C8H8 interconversions. An unusual rearrangement providing a new route to semibullvalene". Journal of the American Chemical Society. 91 (21): 5878. doi:10.1021/ja01049a035.
- Meinwald, Jerrold.; Tsuruta, Haruki. (1970). "(CH)8 hydrocarbons. Photochemistry of tricycloocta-3,7-diene". Journal of the American Chemical Society. 92 (8): 2579. doi:10.1021/ja00711a078.
- Untersuchungen in der Cyclobutanreihe, XII. Zwei stereoisomere Dimere des Cyclobutadiens Margarete Avram, Ilie G. Dinulescu, Elise Marica, Georg Mateescu, Elvira Sliam, Costin D. Nenitzescu Chemische Berichte Volume 97, Issue 2, pages 382–389, February 1964 doi:10.1002/cber.19640970210
- Methyl tetracyclocot-7-ene-4-carboxylate Gerhard W. Klumpp, W. G. J. Rietman, J. J. Vrielink J. Am. Chem. Soc., 1970, 92 (17), pp 5266–5267 doi:10.1021/ja00720a071
- Synthesis and reactions of tetracyclooctanes Leverett R. Smith, George E. Gream, Jerrold Meinwald J. Org. Chem., 1977, 42 (6), pp 927–936 doi:10.1021/jo00426a001
- (Z)-3,7 Bis(phenylsulfonyl)pentacyclooctane, an Octabisvalene Derivative (1985) Angewandte Chemie International Edition in English Volume 24, Issue 5, pages 411–412 doi:10.1002/anie.198504111
- The synthesis of octavalene (tricycloocta-3,5-diene) and several substituted octavalenes Tetrahedron Volume 42, Issue 6, 1986, Pages 1585-1596 Manfred Christl, Reinhard Lang and Clemens Herzog doi:10.1016/S0040-4020(01)87575-X
- Electronic structure of octavalene. Photoelectron spectroscopic investigations Rolf Gleiter, Peter Bischof, Manfred Christl J. Org. Chem., 1986, 51 (15), pp 2895–2898 doi:10.1021/jo00365a007
- E. Vogel, H. Günther (1967). "Benzene Oxide-Oxepin Valence Tautomerism". Angewandte Chemie International Edition in English. 6 (5): 385–401. doi:10.1002/anie.196703851.
External links
 Media related to Valence isomer at Wikimedia Commons
Media related to Valence isomer at Wikimedia Commons