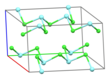
Part of a layer in the crystal structure of YCl3 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Yttrium(III) chloride Yttrium trichloride | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) |
| ||
| ChemSpider | |||
| ECHA InfoCard | 100.030.716 | ||
| EC Number |
| ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | YCl3 | ||
| Molar mass | 195.265 g/mol | ||
| Appearance | white solid | ||
| Density | 2.61 g/cm | ||
| Melting point | 721 °C (1,330 °F; 994 K) | ||
| Boiling point | 1,482 °C (2,700 °F; 1,755 K) | ||
| Solubility in water | 751 g/L (20 °C) | ||
| Solubility | 601 g/L ethanol (15 °C) 606 g/L pyridine (15 °C) | ||
| Structure | |||
| Crystal structure | Monoclinic, mS16 | ||
| Space group | C2/m, No. 12 | ||
| Lattice constant | a = 0.692 nm, b = 1.194 nm, c = 0.644 nmα = 90°, β = 111°, γ = 90° | ||
| Formula units (Z) | 4 | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms | 
| ||
| Signal word | Warning | ||
| Hazard statements | H315, H319, H335 | ||
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P332+P313, P337+P313, P362 | ||
| NFPA 704 (fire diamond) |
 | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
| Other anions | Yttrium(III) fluoride Yttrium(III) bromide Yttrium(III) iodide | ||
| Other cations | Scandium(III) chloride Lutetium(III) chloride | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Yttrium(III) chloride is an inorganic compound of yttrium and chloride. It exists in two forms, the hydrate (YCl3(H2O)6) and an anhydrous form (YCl3). Both are colourless salts that are highly soluble in water and deliquescent.
Structure
Solid YCl3 adopts a cubic structure with close-packed chloride ions and yttrium ions filling one third of the octahedral holes and the resulting YCl6 octahedra sharing three edges with adjacent octahedra, giving it a layered structure. This structure is shared by a range of compounds, notably AlCl3.
Preparation and reactions
YCl3 is often prepared by the "ammonium chloride route," starting from either Y2O3 or hydrated chloride or oxychloride. or YCl3·6H2O. These methods produce (NH4)2:
- 10 NH4Cl + Y2O3 → 2 (NH4)2 + 6 NH3 + 3 H2O
- YCl3·6H2O + 2 NH4Cl → (NH4)2 + 6 H2O
The pentachloride decomposes thermally according to the following equation:
- (NH4)2 → 2 NH4Cl + YCl3
The thermolysis reaction proceeds via the intermediacy of (NH4).
Treating Y2O3 with aqueous HCl produces the hydrated chloride (YCl3·6H2O). When heated, this salt yields yttrium oxychloride rather than reverting to the anhydrous form.
References
- ^ Templeton, D. H.; Carter, Giles F. (1954). "The Crystal Structures of Yttrium Trichloride and Similar Compounds". J. Phys. Chem. 58 (11): 940–944. doi:10.1021/j150521a002.
- ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. p. 4.99. ISBN 978-1439855119.
- Spencer, James F. (1919), The Metals of the Rare Earths, New York: Longmans, Green, and Co, p. 135
- Templeton, D. H.; Carter, Giles F. (1954). "The Crystal Structures of Yttrium Trichloride and Similar Compounds". The Journal of Physical Chemistry. 58 (11): 940–944. doi:10.1021/j150521a002.
- Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- Meyer, G. (1989). "The Ammonium Chloride Route to Anhydrous Rare Earth Chlorides—The Example of Ycl 3". The Ammonium Chloride Route to Anhydrous Rare Earth Chlorides-The Example of YCl3. Inorganic Syntheses. Vol. 25. pp. 146–150. doi:10.1002/9780470132562.ch35. ISBN 978-0-470-13256-2.
- Edelmann, F. T.; Poremba, P. (1997). Herrmann, W. A. (ed.). Synthetic Methods of Organometallic and Inorganic Chemistry. Vol. VI. Stuttgart: Georg Thieme Verlag. ISBN 978-3-13-103021-4.
- Taylor, M.D.; Carter, C.P. (1962). "Preparation of anhydrous lanthanide halides, especially iodides". Journal of Inorganic and Nuclear Chemistry. 24 (4): 387–391. doi:10.1016/0022-1902(62)80034-7.
| Yttrium compounds | |||
|---|---|---|---|
| Yttrium(II) | |||
| Yttrium(III) |
| ||