This is an old revision of this page, as edited by The chemistds (talk | contribs) at 23:23, 10 October 2011 (added CSID, (Std)InChI & (Std)InChIKey). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 23:23, 10 October 2011 by The chemistds (talk | contribs) (added CSID, (Std)InChI & (Std)InChIKey)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Pharmaceutical compound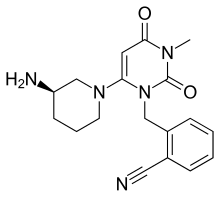 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.256.501 |
| Chemical and physical data | |
| Formula | C18H21N5O2 |
| Molar mass | 339.39 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Alogliptin (codenamed SYR-322) is an investigational anti-diabetic drug in the DPP-4 inhibitor class, being developed by Takeda Pharmaceutical Company. In January 2008, Takeda submitted a New Drug Application for alogliptin to the U.S. Food and Drug Administration, after positive results from Phase III clinical trials. However, the FDA submission was suspended or withdrawn in June 2009 needing more data.
References
- Feng J, Zhang Z, Wallace MB; et al. (2007). "Discovery of alogliptin: a potent, selective, bioavailable, and efficacious inhibitor of dipeptidyl peptidase IV". J. Med. Chem. 50 (10): 2297–300. doi:10.1021/jm070104l. PMID 17441705.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - "Takeda Submits New Drug Application for Alogliptin (SYR-322) in the U.S." (Press release). Takeda Pharmaceutical Company. January 4, 2008. Retrieved 2008-01-09.
- "GEN News Highlights: Takeda Pulls MAA for Type 2 Diabetes Therapy". Genetic Engineering and Biotechnology News. 4 June 2009.
This drug article relating to the gastrointestinal system is a stub. You can help Misplaced Pages by expanding it. |