This is an old revision of this page, as edited by Doc James (talk | contribs) at 17:26, 20 January 2019 (trimmed primary sources). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 17:26, 20 January 2019 by Doc James (talk | contribs) (trimmed primary sources)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) This article is about the chemical. For other uses, see Nicotine (disambiguation).Pharmaceutical compound
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Nicorette, Nicotrol |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Dependence liability | Physical: low–moderate Psychological: moderate–high |
| Addiction liability | High |
| Routes of administration | Inhalation; insufflation; oral – buccal, sublingual, and ingestion; transdermal; rectal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | <5% |
| Metabolism | Primarily hepatic: CYP2A6, CYP2B6, FMO3, others |
| Metabolites | Cotinine |
| Elimination half-life | 1-2 hours; 20 hours active metabolite |
| Excretion | Urine (10-20% (gum), pH-dependent; 30% (inhaled); 10-30% (intranasal)) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.177 |
| Chemical and physical data | |
| Formula | C10H14N2 |
| Molar mass | 162.23 g/mol g·mol |
| 3D model (JSmol) | |
| Chirality | Chiral |
| Density | 1.01 g/cm |
| Melting point | −79 °C (−110 °F) |
| Boiling point | 247 °C (477 °F) |
SMILES
| |
InChI
| |
Nicotine is a potent parasympathomimetic stimulant and an alkaloid found in the nightshade family of plants. Nicotine acts as an exogenous receptor agonist at most nicotinic acetylcholine receptors (nAChRs), except at two nicotinic receptor subunits (nAChRα9 and nAChRα10) where it acts as an exogenous receptor antagonist. Nicotine is found in the leaves of Nicotiana rustica, in amounts of 2–14%; in the tobacco plant, Nicotiana tabacum; in Duboisia hopwoodii; and in Asclepias syriaca.
It constitutes approximately 0.6–3.0% of the dry weight of tobacco. Usually consistent concentrations of nicotine varying from 2–7 µg/kg (20–70 millionths of a percent wet weight) are found in the edible family Solanaceae, such as potatoes, tomatoes, and eggplant. Some research indicates that the contribution of nicotine obtained from food is substantial in comparison to inhalation of second-hand smoke. Others consider nicotine obtained from food to be trivial unless exceedingly high amounts of certain vegetables are eaten. It functions as an antiherbivore chemical; consequently, nicotine was widely used as an insecticide in the past, and neonicotinoids, such as imidacloprid, are widely used.
Nicotine is highly addictive. It is one of the most commonly abused drugs. An average cigarette yields about 2 mg of absorbed nicotine, while high amounts (30–60 mg) can be harmful. Nicotine induces both behavioral stimulation and anxiety in animals. Nicotine addiction involves drug-reinforced behavior, compulsive use, and relapse following abstinence. Nicotine dependence involves tolerance, sensitization, physical dependence, and psychological dependence. Nicotine dependency causes distress. Nicotine withdrawal symptoms include depressed mood, stress, anxiety, irritability, difficulty concentrating, and sleep disturbances. Mild nicotine withdrawal symptoms are measurable in unrestricted smokers, who experience normal moods only as their blood nicotine levels peak, with each cigarette. On quitting, withdrawal symptoms worsen sharply, then gradually improve to a normal state.
The health effects of long-term nicotine use are unknown. The general medical position is that nicotine itself poses few health risks, except among certain vulnerable groups, such as youth. Nicotine use as a tool for quitting smoking has a good safety history. Nicotine use in the form of nicotine replacement products poses less of a cancer risk than smoking. There is inadequate data to establish if nicotine is itself a carcinogen, but there is evidence of possible risks. Nicotine is linked to possible birth defects. Nicotine use during pregnancy increases the child's risk of type 2 diabetes, obesity, hypertension, neurobehavioral defects, respiratory dysfunction, and infertility. Nicotine is potentially lethal and at sufficiently high doses, it is associated with poisonings. The use of electronic cigarettes, which are designed to be refilled with nicotine-containing e-liquid, has raised concerns over nicotine overdoses, especially with regard to the possibility of young children ingesting the liquids.
Uses
Medical
Main article: Nicotine replacement therapy
The primary therapeutic use of nicotine is in treating nicotine dependence in order to eliminate smoking with the damage it does to health. Controlled levels of nicotine are given to patients through gums, dermal patches, lozenges, electronic/substitute cigarettes or nasal sprays in an effort to wean them off their dependence. Studies have found that these therapies increase the chance of success of quitting by 50 to 70%, though reductions in the population as a whole have not been demonstrated.
In contrast to recreational nicotine products, which have have been designed to maximize the likelihood of addiction, nicotine replacement products (NRTs) are designed to minimize addictiveness. The more quickly a dose of nicotine is delivered and absorbed, the higher the addiction risk. Some forms of NRT deliver nicotine more quickly than others, and it is possible to become dependent on some NRTs.
Pesticide
Nicotine has been used as an insecticide since at least the 1690s, in the form of tobacco extracts (although other components of tobacco also seem to have pesticide effects). Nicotine pesticides have not been commercially available in the US since 2014, and homemade pesticides are banned on organic crops and counterrecommended for small gardeners. Nicotine pesticides have been banned in the EU since 2009. Foods are imported from countries in which nicotine pesticides are allowed, such as China, but foods may not exceed maximum nicotine levels. Neonicotinoids, which are derived from and structurally similar to nicotine, are widely used as agricultural and veterinary pesticides as of 2016.
In nicotine-producing plants, nicotine functions as an antiherbivory chemical; consequently, nicotine has been widely used as an insecticide, and neonicotinoids, such as imidacloprid, are widely used.
Enhancing performance
Nicotine-containing products are sometimes used for the performance-enhancing effects of nicotine on cognition. A meta-analysis of 41 double-blind, placebo-controlled studies concluded that nicotine or smoking had significant positive effects on aspects of fine motor abilities, alerting and orienting attention, and episodic and working memory. A 2015 review noted that stimulation of the α4β2 nicotinic receptor is responsible for certain improvements in attentional performance; among the nicotinic receptor subtypes, nicotine has the highest binding affinity at the α4β2 receptor (ki=1 nM), which is also the biological target that mediates nicotine's addictive properties. Nicotine has potential beneficial effects, but it also has paradoxical effects, which may be due to the inverted U-shape of the dose-response curve or pharmacokinetic features.
Recreational
Nicotine is used as a recreational drug. It is widely used because it is highly addictive and hard to discontinue using it. Nicotine is normally used compulsively, and dependence can develop within days. Recreational drug users commonly use nicotine for its mood-altering effects. Other recreational nicotine products include chewing tobacco, cigars, cigarettes, e-cigarettes, snuff, pipe tobacco, and snus.
Adverse effects
Further information: Safety of electronic cigarettes § NicotineNicotine is not completely harmless, but it is safer than inhaled tobacco smoke. It has effects on multiple organ systems. The health effects of long-term nicotine use are unknown. A 2010 World Health Organization report states, "Addiction to tobacco kills one person prematurely every six seconds. One in two long-term smokers—largely in low- and middle-income countries—will die from tobacco addiction. This epidemic reflects the highly addictive nature of tobacco, and specifically of nicotine, its principal addicting component." The long-term health effects of nicotine in the form of vapor is unknown. It is hard to determine the long-term safety of nicotine. Data on the long-term use of nicotine is relatively limited. Nicotine exposure regardless of the duration has not been found to be dangerous in adults. Limited data exists on the health effects of long-term use of pure nicotine, because nicotine is mostly consumed via tobacco products.
The long-term use of nicotine in the form of snus incurs a slight risk of cardiovascular disease compared to tobacco smoking and there is evidence of an increased risk in snus users of heart failure. The available literature strengthens the evidence that any cancer risk (including that of pancreatic cancer) is at most minimal. The complex effects of nicotine are not entirely understood. Some studies of continued use of nicotine replacement products in those who have stopped smoking found no adverse effects from months to several years, and other studies suggest that people with cardiovascular disease were able to tolerate them for up to 12 weeks.
The accepted medical position in 2007 was that nicotine itself poses few health risks, except among certain vulnerable groups. In 2016, Royal College of Physicians' report "argued that nicotine use, of itself, presents relatively little risk to users or wider society," and the ideal course of action for smokers is to quit all nicotine use. Adolescents seems to be vulnerable to the negative effects of nicotine on the central nervous system. Youth are especially vulnerable to nicotine's effects on the growing neural tissue. A 2014 American Heart Association policy statement found that some health concerns relate to nicotine. These concerns are associated with nicotine being able to facilitate the release of catecholamines, including hemodynamic effects, etc. Experimental research suggests that adolescent nicotine use may harm brain development. Nicotine use through vaping is harmful for children. Maternal nicotine intake may result in a number of lifelong health issues for the child. As medicine, nicotine is used to help with quitting smoking and has good safety in this form.
Immune system
Immune cells of both innate and acquired immune subsystems frequently express the α2, α5, α6, α7, α9, and α10 subunits of nicotinic acetylcholine receptors. Evidence suggests these receptors, whose ligand is nicotine, are involved in the regulation of immune function. It is not yet clear to what extent short-term or long-term regular systemic administration of nicotine disrupts the immune function via receptor signaling. However, a 2017 study discovered that both pure nicotine and nicotine from cigarette smoke binding receptors impair macrophage killing of deadly global human pathogenic bacteria.
Metabolism and body weight
Further information: Cigarette smoking for weight lossBy reducing the appetite and raising the metabolism, a certain number of smokers may lose weight as a consequence. By increasing metabolic rate and inhibiting the usual compensatory increase in appetite, the body weight of smokers is lower on average than that of non-smokers. When smokers quit, they gain on average 5–6 kg weight, returning to the average weight of non-smokers. The evidence suggests that heavy smokers tend to gain more weight compared with light smokers. The factors causing heavy smokers to tend to gain more weight than light smokers or non-smokers has not been resolved.
Vascular system
A 2014 review found that nicotine use is not a significant cause of cardiovascular disease. A 2015 review found that nicotine is associated with cardiovascular disease. Nicotine could trigger atrial fibrillation and other abnormal cardiovascular events, such as the acute myocardial infarction in a previously healthy man. Additionally, nicotine has potential vasoconstrictive effects on the brain. Snus use is associated with a somewhat reduced risk of non-fatal acute myocardial infarction, but with an increased risk of fatal acute myocardial infarction. A 2016 review suggests that "the risks of nicotine without tobacco combustion products (cigarette smoke) are low compared to cigarette smoking, but are still of concern in people with cardiovascular disease." Some studies in people show the possibility that nicotine contributes to acute cardiovascular events in smokers with established cardiovascular disease, and induces pharmacologic effects that might contribute to increased atherosclerosis. Prolonged nicotine use seems not to increase atherosclerosis. Brief nicotine use, such as nicotine medicine, seems to incur a slight cardiovascular risk, even to people with established cardiovascular disease. A 2015 review found "Nicotine in vitro and in animal models can inhibit apoptosis and enhance angiogenesis, effects that raise concerns about the role of nicotine in promoting the acceleration of atherosclerotic disease." A 2012 Cochrane review found no evidence of an increased risk of cardiovascular disease with nicotine replacement products. A 1996 randomized controlled trial using nicotine patches found that serious adverse events were not more frequent among smokers with cardiovascular disease. A meta-analysis shows that snus consumption, which delivers nicotine at a dose equivalent to that of cigarettes, is not associated with heart attacks. Hence, it is not nicotine, but tobacco smoke's other components which seem to be implicated in ischemic heart disease. Nicotine increases heart rate and blood pressure and induces abnormal heart rhythms. Nicotine can also induce potentially atherogenic genes in human coronary artery endothelial cells. Microvascular injury can result through its action on nicotinic acetylcholine receptors (nAChRs). Nicotine does not adversely affect serum cholesterol levels, but a 2015 review found it may elevate serum cholesterol levels. Many quitting smoking studies using nicotine medicines report lowered dyslipidemia with considerable benefit in HDL/LDL ratios. Nicotine supports clot formation and aids in plaque formation by enhancing vascular smooth muscle.
Endocrine system
Nicotine causes various endocrine imbalances, and has negative effects on pituitary, thyroid, adrenal, testicular and ovarian functions.
Cancer

Although there is insufficient evidence to classify nicotine as a carcinogen, there is an ongoing debate about whether it functions as a tumor promoter. In vitro studies have associated it with cancer, but carcinogenicity has not been demonstrated in vivo. There is inadequate research to demonstrate that nicotine is associated with cancer in humans, but there is evidence indicating possible oral, esophageal, or pancreatic cancer risks. Nicotine can induce inflammation in the lungs that imitates metastatic cancer. Nicotine in the form of nicotine replacement products poses less of a cancer risk than smoking. Nicotine replacement products have not been shown to be associated with cancer in the real world. Nicotine exerts DNA damage in the Escherichia colipol A+/pol− test. Low levels of nicotine stimulate cell proliferation, while high levels are cytotoxic.
While no epidemiological evidence directly supports the notion that nicotine acts as a carcinogen in the formation of human cancer, research has identified nicotine's indirect involvement in cancer formation in animal models and cell cultures. Nicotine increases cholinergic signalling and adrenergic signalling in the case of colon cancer, thereby impeding apoptosis (programmed cell death), promoting tumor growth, and activating growth factors and cellular mitogenic factors such as 5-lipoxygenase (5-LOX), and epidermal growth factor (EGF). Nicotine also promotes cancer growth by stimulating angiogenesis and neovascularization. In one study, nicotine administered to mice with tumors caused increases in tumor size (twofold increase), metastasis (nine-fold increase), and tumor recurrence (threefold increase). N-Nitrosonornicotine (NNN), classified by the International Agency for Research on Cancer (IARC) as a Group 1 carcinogen, has been shown to form in vitro from nornicotine in human saliva, indicating nornicotine is a carcinogen precursor. The IARC has not evaluated pure nicotine or assigned it to an official carcinogenic classification.
In cancer cells, nicotine promotes the epithelial–mesenchymal transition which makes the cancer cells more resistant to drugs that treat cancer.
Sleep
Nicotine dependence is associated with poor sleep quality and shorter sleep duration among smokers. Nicotine itself causes insomnia-like sleep changes in healthy non-smokers. At the molecular level, nicotine binding acetylcholine receptors engage in modulation of wakefulness. A 2017 sleep-wake cycle related hypothesis based on murine research implicates the nicotine binding alpha-9 cholinergic receptor subunit within retinal signaling processes in positive masking of sleep by light, that is, in using light to promote the incremental wake drifting in the 24-hour sleep-wake cycle.
Fetal development and breastfeeding
Pregnant women, breastfeeding mothers, and the elderly are more sensitive to nicotine than other individuals. Nicotine is not safe to use in any amount during pregnancy. Nicotine crosses the placenta and is found in the breast milk of mothers who smoke as well as mothers who inhale passive smoke. Nicotine accumulates in the fetus because it goes through the placenta.
Strong evidence suggests that nicotine cannot be regarded as a safe substance of cigarette use. Nicotine itself could be at least partly responsible for many of the adverse after birth health results related to cigarette use while the mother was pregnant. The use of any nicotine-containing products during pregnancy probably will result in adverse consequences of fetal brain growth. There is evidence that nicotine negatively affects fetal brain development and pregnancy outcomes. There is also a risk of stillbirth and pre-term birth. Risks to the child later in life from nicotine exposure during pregnancy include type 2 diabetes, obesity, hypertension, neurobehavioral defects, respiratory dysfunction, and infertility.
In pregnancy, a 2013 review noted that "Although the exact mechanisms by which nicotine produces adverse fetal effects are unknown, it is likely that hypoxia, undernourishment of the fetus, and direct vasoconstrictor effects on the placental and umbilical vessels all play a role. Nicotine also has been shown to have significant deleterious effects on brain development, including alterations in brain metabolism and neurotransmitter systems and abnormal brain development." It also notes that "abnormalities of newborn neurobehavior, including impaired orientation and autonomic regulation and abnormalities of muscle tone, have been identified in a number of prenatal nicotine exposure studies", that there is weak data associating fetal nicotine exposure with newborn facial clefts, that there is no good evidence for newborns suffering nicotine withdrawal from fetal exposure to nicotine, and that "nicotine is only 1 of more than 4000 compounds to which the fetus is exposed through maternal smoking. Of these, ∼30 compounds have been associated with adverse health outcomes".
Effective 1 April 1990, the Office of Environmental Health Hazard Assessment (OEHHA) of the California Environmental Protection Agency added nicotine to the list of chemicals known to cause developmental toxicity.
Use of other drugs
Main article: Tobacco and other drugs See also: Gateway drug theoryIn animals, it is relatively simple to determine if consumption of a certain drug increases the later attraction of another drug. In humans, where such direct experiments are not possible, longitudinal studies can show if the probability of a substance use is related to the earlier use of other substances.
In mice nicotine increased the probability of later consumption of cocaine and the experiments permitted concrete conclusions on the underlying molecular biological alteration in the brain. The biological changes in mice correspond to the epidemiological observations in humans that nicotine consumption is coupled to an increased probability of later use of cannabis and cocaine.
In rats cannabis consumption – earlier in life – increased the later self-administration of nicotine. A 2012 study of drug use of 14,577 US 12th graders showed that alcohol consumption was associated with an increased probability of later use of tobacco, cannabis, and illegal drugs.
Overdose
Main article: Nicotine poisoningNicotine is regarded as a potentially lethal poison. The LD50 of nicotine is 50 mg/kg for rats and 3 mg/kg for mice. 30–60 mg (0.5–1.0 mg/kg) can be a lethal dosage for adult humans. However, the widely used human LD50 estimate of 0.5–1.0 mg/kg was questioned in a 2013 review, in light of several documented cases of humans surviving much higher doses; the 2013 review suggests that the lower limit causing fatal outcomes is 500–1000 mg of ingested nicotine, corresponding to 6.5–13 mg/kg orally. Nevertheless, nicotine has a relatively high toxicity in comparison to many other alkaloids such as caffeine, which has an LD50 of 127 mg/kg when administered to mice.
At high-enough doses, it is associated with nicotine poisoning. Today nicotine is less commonly used in agricultural insecticides, which was a main source of poisoning. More recent cases of poisoning typically appear to be in the form of Green Tobacco Sickness or due to accidental ingestion of tobacco or tobacco products or ingestion of nicotine-containing plants. People who harvest or cultivate tobacco may experience Green Tobacco Sickness (GTS), a type of nicotine poisoning caused by dermal exposure to wet tobacco leaves. This occurs most commonly in young, inexperienced tobacco harvesters who do not consume tobacco. People can be exposed to nicotine in the workplace by breathing it in, skin absorption, swallowing it, or eye contact. The Occupational Safety and Health Administration (OSHA) has set the legal limit (permissible exposure limit) for nicotine exposure in the workplace as 0.5 mg/m skin exposure over an 8-hour workday. The US National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 0.5 mg/m skin exposure over an 8-hour workday. At environmental levels of 5 mg/m, nicotine is immediately dangerous to life and health.
It is unlikely that a person would overdose on nicotine through smoking alone. The US Food and Drug Administration (FDA) stated in 2013 that "There are no significant safety concerns associated with using more than one OTC NRT at the same time, or using an OTC NRT at the same time as another nicotine-containing product—including a cigarette."
The rise in the use of electronic cigarettes, many forms of which are designed to be refilled with nicotine-containing e-liquid supplied in small plastic bottles, has raised concerns over nicotine overdoses, especially in the possibility of young children ingesting the liquids. A 2015 Public Health England report noted an "unconfirmed newspaper report of a fatal poisoning of a two-year old child" and two published case reports of children of similar age who had recovered after ingesting e-liquid and vomiting. They also noted case reports of suicides by nicotine. Where adults drank liquid containing up to 1,500 mg of nicotine they recovered (helped by vomiting), but an ingestion apparently of about 10,000 mg was fatal, as was an injection. They commented that "Serious nicotine poisoning seems normally prevented by the fact that relatively low doses of nicotine cause nausea and vomiting, which stops users from further intake." The FDA recommends that e-cigarettes and e-liquids be kept in a safe place, where children and pets do not have access to them.
Reinforcement disorders
See also: Nicotine withdrawal and Smoking cessation
Nicotine is highly addictive, comparable to heroin or cocaine. Nicotine dependence involves aspects of both psychological dependence and physical dependence, since discontinuation of extended use has been shown to produce both affective (e.g., anxiety, irritability, craving, anhedonia) and somatic (mild motor dysfunctions such as tremor) withdrawal symptoms. Withdrawal symptoms peak in one to three days and can persist for several weeks. Some people experience symptoms for 6 months or longer.
Normal between-cigarettes discontinuation, in unrestricted smokers, causes mild but measurable nicotine withdrawal symptoms. These include mildly worse mood, stress, anxiety, cognition, and sleep, all of which briefly return to normal with the next cigarette. Smokers have worse mood than they would have if they were not nicotine-dependent; they experience normal moods only immediately after smoking.
There is no clear evidence of cognitive effects of nicotine in nonabstinent smokers or healthy older nonsmokers, but in dependent smokers, withdrawal causes worse cognition, and smoking during withdrawal returns cognitive abilities to pre-withdrawal levels. The temporarily increased cognitive levels of smokers after inhaling smoke are offset by periods of cognitive decline during nicotine withdrawal. Therefore, the overall daily cognitive levels of smokers and non-smokers are roughly similar.
Nicotine activates the mesolimbic pathway and induces long-term ΔFosB expression in the nucleus accumbens when inhaled or injected at sufficiently high doses, but not necessarily when ingested. Consequently, repeated daily exposure (possibly excluding oral route) to nicotine can result in accumbal ΔFosB overexpression, in turn causing nicotine addiction.
Pharmacology
Nicotine's mood-altering effects are different by report: in particular it is both a stimulant and a relaxant. First causing a release of glucose from the liver and epinephrine (adrenaline) from the adrenal medulla, it causes stimulation. Users report feelings of relaxation, sharpness, calmness, and alertness.
When a cigarette is smoked, nicotine-rich blood passes from the lungs to the brain within seven seconds and immediately stimulates nicotinic acetylcholine receptors; this indirectly promotes the release of many chemical messengers such as acetylcholine, norepinephrine, epinephrine, arginine vasopressin, serotonin, dopamine, and beta-endorphin in parts of the brain. Nicotine also increases the sensitivity of the brain's reward system to rewarding stimuli. An average cigarette yields about 2 mg of absorbed nicotine. Studies suggest that when smokers wish to achieve a stimulating effect, they take short quick puffs, which produce a low level of blood nicotine.
Nicotine is unusual in comparison to most drugs, as its profile changes from stimulant to sedative with increasing dosages, a phenomenon known as "Nesbitt's paradox" after the doctor who first described it in 1969. At very high doses it dampens neuronal activity. Nicotine induces both behavioral stimulation and anxiety in animals.
Pharmacodynamics
Nicotine acts as a receptor agonist at most nicotinic acetylcholine receptors (nAChRs), except at two nicotinic receptor subunits (nAChRα9 and nAChRα10) where it acts as a receptor antagonist.
Central nervous system

By binding to nicotinic acetylcholine receptors in the brain, nicotine elicits its psychoactive effects and increases the levels of several neurotransmitters in various brain structures – acting as a sort of "volume control."
| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Nicotine" – news · newspapers · books · scholar · JSTOR (March 2016) (Learn how and when to remove this message) |
Nicotine has a higher affinity for nicotinic receptors in the brain than those in skeletal muscle, though at toxic doses it can induce contractions and respiratory paralysis. Nicotine's selectivity is thought to be due to a particular amino acid difference on these receptor subtypes.
Nicotine activates nicotinic receptors (particularly α4β2 nicotinic receptors) on neurons that innervate the ventral tegmental area and within the mesolimbic pathway where it appears to cause the release of dopamine. This nicotine-induced dopamine release occurs at least partially through activation of the cholinergic–dopaminergic reward link in the ventral tegmental area. Nicotine also appears to induce the release of endogenous opioids that activate opioid pathways in the reward system, since naltrexone – an opioid receptor antagonist – blocks nicotine self-administration. These actions are largely responsible for the strongly reinforcing effects of nicotine, which often occur in the absence of euphoria; however, mild euphoria from nicotine use can occur in some individuals. Chronic nicotine use inhibits class I and II histone deacetylases in the striatum, where this effect plays a role in nicotine addiction.
Sympathetic nervous system
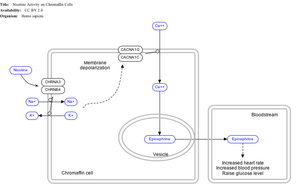
Nicotine also activates the sympathetic nervous system, acting via splanchnic nerves to the adrenal medulla, stimulating the release of epinephrine. Acetylcholine released by preganglionic sympathetic fibers of these nerves acts on nicotinic acetylcholine receptors, causing the release of epinephrine (and norepinephrine) into the bloodstream.
Adrenal medulla
By binding to ganglion type nicotinic receptors in the adrenal medulla, nicotine increases flow of adrenaline (epinephrine), a stimulating hormone and neurotransmitter. By binding to the receptors, it causes cell depolarization and an influx of calcium through voltage-gated calcium channels. Calcium triggers the exocytosis of chromaffin granules and thus the release of epinephrine (and norepinephrine) into the bloodstream. The release of epinephrine (adrenaline) causes an increase in heart rate, blood pressure and respiration, as well as higher blood glucose levels.
Pharmacokinetics

As nicotine enters the body, it is distributed quickly through the bloodstream and crosses the blood–brain barrier reaching the brain within 10–20 seconds after inhalation. The elimination half-life of nicotine in the body is around two hours.
The amount of nicotine absorbed by the body from smoking can depend on many factors, including the types of tobacco, whether the smoke is inhaled, and whether a filter is used. However, it has been found that the nicotine yield of individual products has only a small effect (4.4%) on the blood concentration of nicotine, suggesting "the assumed health advantage of switching to lower-tar and lower-nicotine cigarettes may be largely offset by the tendency of smokers to compensate by increasing inhalation".
Nicotine has a half-life of 1–2 hours. Cotinine is an active metabolite of nicotine that remains in the blood with a half-life of 18–20 hours, making it easier to analyze.
Nicotine is metabolized in the liver by cytochrome P450 enzymes (mostly CYP2A6, and also by CYP2B6) and FMO3, which selectively metabolizes (S)-nicotine. A major metabolite is cotinine. Other primary metabolites include nicotine N'-oxide, nornicotine, nicotine isomethonium ion, 2-hydroxynicotine and nicotine glucuronide. Under some conditions, other substances may be formed such as myosmine.
Glucuronidation and oxidative metabolism of nicotine to cotinine are both inhibited by menthol, an additive to mentholated cigarettes, thus increasing the half-life of nicotine in vivo.
Chemistry
| NFPA 704 safety square | |
|---|---|
 |
Nicotine is a hygroscopic, colorless to yellow-brown, oily liquid, that is readily soluble in alcohol, ether or light petroleum. It is miscible with water in its base form between 60 °C and 210 °C. As a nitrogenous base, nicotine forms salts with acids that are usually solid and water-soluble. Its flash point is 95 °C and its auto-ignition temperature is 244 °C.
Nicotine is readily volatile (vapor pressure 5.5 ㎩ at 25 ℃) and dibasic (Kb1=1×10⁻⁶, Kb2=1×10⁻¹¹).
Nicotine is optically active, having two enantiomeric forms. The naturally occurring form of nicotine is levorotatory with a specific rotation of D=–166.4° ((−)-nicotine). The dextrorotatory form, (+)-nicotine is physiologically less active than (−)-nicotine. (−)-nicotine is more toxic than (+)-nicotine. The salts of (+)-nicotine are usually dextrorotatory. The hydrochloride and sulphate salts become optically inactive if heated in a closed vessel above 180 °C.
On exposure to ultraviolet light or various oxidizing agents, nicotine is converted to nicotine oxide, nicotinic acid (vitamin B3), and methylamine.
Occurrence and biosynthesis

Nicotine is a natural product of tobacco, occurring in the leaves in a range of 0.5 to 7.5% depending on variety. Nicotine also naturally occurs in smaller amounts in plants from the family Solanaceae (such as potatoes, tomatoes, eggplant, and peppers). The amounts of nicotine of tomato varieties lowered substantially as the fruits ripened. Nicotine content in tea leaves is greatly inconsistent and in some cases considerably greater than in the Solanaceae fruits. A 1999 report found "In some papers it is suggested that the contribution of dietary nicotine intake is significant when compared with exposure to ETS or by active smoking of small numbers of cigarettes. Others consider the dietary intake to be negligible unless inordinately large amounts of specific vegetables are consumed." The amount of nicotine eaten per day is roughly around 1.4 and 2.25 µg/day at the 95th percentile. These numbers may be low due to insufficient food intake data. Since the amounts of nicotine from the Solanum family including potato, tomato, eggplant, and from the Capsicum family vary in the parts per billion, they are tough to measure.
The biosynthetic pathway of nicotine involves a coupling reaction between the two cyclic structures that compose nicotine. Metabolic studies show that the pyridine ring of nicotine is derived from niacin (nicotinic acid) while the pyrrolidone is derived from N-methyl-Δ-pyrrollidium cation. Biosynthesis of the two component structures proceeds via two independent syntheses, the NAD pathway for niacin and the tropane pathway for N-methyl-Δ-pyrrollidium cation.
The NAD pathway in the genus Nicotiana begins with the oxidation of aspartic acid into α-imino succinate by aspartate oxidase (AO). This is followed by a condensation with glyceraldehyde-3-phosphate and a cyclization catalyzed by quinolinate synthase (QS) to give quinolinic acid. Quinolinic acid then reacts with phosphoriboxyl pyrophosphate catalyzed by quinolinic acid phosphoribosyl transferase (QPT) to form niacin mononucleotide (NaMN). The reaction now proceeds via the NAD salvage cycle to produce niacin via the conversion of nicotinamide by the enzyme nicotinamidase.
The N-methyl-Δ-pyrrollidium cation used in the synthesis of nicotine is an intermediate in the synthesis of tropane-derived alkaloids. Biosynthesis begins with decarboxylation of ornithine by ornithine decarboxylase (ODC) to produce putrescine. Putrescine is then converted into N-methyl putrescine via methylation by SAM catalyzed by putrescine N-methyltransferase (PMT). N-methylputrescine then undergoes deamination into 4-methylaminobutanal by the N-methylputrescine oxidase (MPO) enzyme, 4-methylaminobutanal then spontaneously cyclize into N-methyl-Δ-pyrrollidium cation.
The final step in the synthesis of nicotine is the coupling between N-methyl-Δ-pyrrollidium cation and niacin. Although studies conclude some form of coupling between the two component structures, the definite process and mechanism remains undetermined. The current agreed theory involves the conversion of niacin into 2,5-dihydropyridine through 3,6-dihydronicotinic acid. The 2,5-dihydropyridine intermediate would then react with N-methyl-Δ-pyrrollidium cation to form enantiomerically pure (−)-nicotine.
Detection in body fluids
Nicotine can be quantified in blood, plasma, or urine to confirm a diagnosis of poisoning or to facilitate a medicolegal death investigation. Urinary or salivary cotinine concentrations are frequently measured for the purposes of pre-employment and health insurance medical screening programs. Careful interpretation of results is important, since passive exposure to cigarette smoke can result in significant accumulation of nicotine, followed by the appearance of its metabolites in various body fluids. Nicotine use is not regulated in competitive sports programs.
History
See also: History of tobaccoNicotine is named after the tobacco plant Nicotiana tabacum, which in turn is named after the French ambassador in Portugal, Jean Nicot de Villemain, who sent tobacco and seeds to Paris in 1560, presented to the French King, and who promoted their medicinal use. Smoking was believed to protect against illness, particularly the plague.
Tobacco was introduced to Europe in 1559, and by the late 17th century, it was used not only for smoking but also as an insecticide. After World War II, over 2,500 tons of nicotine insecticide were used worldwide, but by the 1980s the use of nicotine insecticide had declined below 200 tons. This was due to the availability of other insecticides that are cheaper and less harmful to mammals.
Currently, nicotine, even in the form of tobacco dust, is prohibited as a pesticide for organic farming in the United States.
In 2008, the EPA received a request, from the registrant, to cancel the registration of the last nicotine pesticide registered in the United States. This request was granted, and since 1 January 2014, this pesticide has not been available for sale.
US FDA Commissioner Scott Gottlieb, M.D., announced on 28 July 2017 a comprehensive regulatory plan for tobacco and nicotine regulation that will serve as a multi-year roadmap to better protect kids and significantly reduce tobacco-related disease and death, including pursuing lowering nicotine in regular cigarettes to a minimally or non-addictive level. Nicotine is one of the most rigorously studied drugs.
Chemical identification
Nicotine was originally isolated from the tobacco plant in 1828 by chemists Wilhelm Heinrich Posselt and Karl Ludwig Reimann from Germany, who believed it was a poison. Its chemical empirical formula was described by Melsens in 1843, its structure was discovered by Adolf Pinner and Richard Wolffenstein in 1893, and it was first synthesized by Amé Pictet and A. Rotschy in 1904.
Society and culture
The nicotine content of popular American-brand cigarettes has increased over time, and one study found that there was an average increase of 1.78% per year between the years of 1998 and 2005.
Research
While acute/initial nicotine intake causes activation of nicotine receptors, chronic low doses of nicotine use leads to desensitisation of nicotine receptors (due to the development of tolerance) and results in an antidepressant effect, with early research showing low dose nicotine patches could be an effective treatment of major depressive disorder in non-smokers. However, the original research concluded that: "Nicotine patches produced short-term improvement of depression with minor side effects. Because of nicotine's high risk to health, nicotine patches are not recommended for clinical use in depression."
Though tobacco smoking is associated with an increased risk of Alzheimer's disease, there is evidence that nicotine itself has the potential to prevent and treat Alzheimer's disease.
Research into nicotine's most predominant metabolite, cotinine, suggests that some of nicotine's psychoactive effects are mediated by cotinine.
Little research is available in humans but animal research suggests there is potential benefit from nicotine in Parkinson's disease. In humans, there is epidemiologic evidence for a reduced risk of Parkinson's associated with tobacco use, consumption of Solanaceae vegetables in general, and consumption of peppers in particular.
See also
- ABT-418
- Anabasine
- Cytisine
- Nicotiana rustica
- Nicotiana tabacum
- Lobelia inflata
- Substance dependence
- Tobacco products
References
- ^ D'Souza MS, Markou A (July 2011). "Neuronal mechanisms underlying development of nicotine dependence: implications for novel smoking-cessation treatments". Addiction Science & Clinical Practice. 6 (1): 4–16. PMC 3188825. PMID 22003417.
Withdrawal symptoms upon cessation of nicotine intake: Chronic nicotine use induces neuroadaptations in the brain's reward system that result in the development of nicotine dependence. Thus, nicotine-dependent smokers must continue nicotine intake to avoid distressing somatic and affective withdrawal symptoms. Newly abstinent smokers experience symptoms such as depressed mood, anxiety, irritability, difficulty concentrating, craving, bradycardia, insomnia, gastrointestinal discomfort, and weight gain (Shiffman and Jarvik, 1976; Hughes et al., 1991). Experimental animals, such as rats and mice, exhibit a nicotine withdrawal syndrome that, like the human syndrome, includes both somatic signs and a negative affective state (Watkins et al., 2000; Malin et al., 2006). The somatic signs of nicotine withdrawal include rearing, jumping, shakes, abdominal constrictions, chewing, scratching, and facial tremors. The negative affective state of nicotine withdrawal is characterized by decreased responsiveness to previously rewarding stimuli, a state called anhedonia.
- Cosci F, Pistelli F, Lazzarini N, Carrozzi L (2011). "Nicotine dependence and psychological distress: outcomes and clinical implications in smoking cessation". Psychology Research and Behavior Management. 4: 119–28. doi:10.2147/prbm.s14243. PMC 3218785. PMID 22114542.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Hollinger, Mannfred A. (19 October 2007). Introduction to Pharmacology (Third ed.). Abingdon: CRC Press. pp. 222–223. ISBN 978-1-4200-4742-4.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- Abou-Donia, Mohamed (5 February 2015). Mammalian Toxicology. John Wiley & Sons. pp. 587–. ISBN 978-1-118-68285-2.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Nicotinic acetylcholine receptors: Introduction". IUPHAR Database. International Union of Basic and Clinical Pharmacology. Retrieved 1 September 2014.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 9: Autonomic Nervous System". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. p. 234. ISBN 9780071481274.
Nicotine ... is a natural alkaloid of the tobacco plant. Lobeline is a natural alkaloid of Indian tobacco. Both drugs are agonists are nicotinic cholinergic receptors ...
- ^ Kishioka S, Kiguchi N, Kobayashi Y, Saika F (2014). "Nicotine effects and the endogenous opioid system". Journal of Pharmacological Sciences. 125 (2): 117–24. PMID 24882143.
- ^ Metcalf, Robert L. (2007), "Insect Control", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 9
{{citation}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - "Smoking and Tobacco Control Monograph No. 9" (PDF). Retrieved 19 December 2012.
- ^ Siegmund B, Leitner E, Pfannhauser W (August 1999). "Determination of the nicotine content of various edible nightshades (Solanaceae) and their products and estimation of the associated dietary nicotine intake". Journal of Agricultural and Food Chemistry. 47 (8): 3113–20. doi:10.1021/jf990089w. PMID 10552617.
- Rodgman, Alan; Perfetti, Thomas A. (2009). The chemical components of tobacco and tobacco smoke. Boca Raton, FL: CRC Press. ISBN 978-1-4200-7883-1. LCCN 2008018913.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Ujváry, István (1999). "Nicotine and Other Insecticidal Alkaloids". In Yamamoto, Izuru; Casida, John (eds.). Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor. Tokyo: Springer-Verlag. pp. 29–69.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Grana R, Benowitz N, Glantz SA (May 2014). "E-cigarettes: a scientific review". Circulation. 129 (19): 1972–86. doi:10.1161/circulationaha.114.007667. PMC 4018182. PMID 24821826.
- ^ Holbrook BD (June 2016). "The effects of nicotine on human fetal development". Birth Defects Research. Part C, Embryo Today. 108 (2): 181–92. doi:10.1002/bdrc.21128. PMID 27297020.
- ^ Siqueira LM (January 2017). "Nicotine and Tobacco as Substances of Abuse in Children and Adolescents". Pediatrics. 139 (1): e20163436. doi:10.1542/peds.2016-3436. PMID 27994114.
- Sajja RK, Rahman S, Cucullo L (March 2016). "Drugs of abuse and blood-brain barrier endothelial dysfunction: A focus on the role of oxidative stress". Journal of Cerebral Blood Flow and Metabolism. 36 (3): 539–54. doi:10.1177/0271678X15616978. PMC 4794105. PMID 26661236.
- ^ Mayer B (January 2014). "How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century". Archives of Toxicology. 88 (1): 5–7. doi:10.1007/s00204-013-1127-0. PMC 3880486. PMID 24091634.
- ^ "Nicotine (PIM)". Inchem.org. Retrieved 19 December 2012.
- Caponnetto P, Campagna D, Papale G, Russo C, Polosa R (February 2012). "The emerging phenomenon of electronic cigarettes". Expert Review of Respiratory Medicine. 6 (1): 63–74. doi:10.1586/ers.11.92. PMID 22283580.
- Jain R, Mukherjee K, Balhara YP (April 2008). "The role of NMDA receptor antagonists in nicotine tolerance, sensitization, and physical dependence: a preclinical review". Yonsei Medical Journal. 49 (2): 175–88. doi:10.3349/ymj.2008.49.2.175. PMC 2615322. PMID 18452252.
- Miyasato K (March 2013). "". Nihon Rinsho. Japanese Journal of Clinical Medicine. 71 (3): 477–81. PMID 23631239.
- ^ Parrott AC (July 2015). "Why all stimulant drugs are damaging to recreational users: an empirical overview and psychobiological explanation". Human Psychopharmacology. 30 (4): 213–24. doi:10.1002/hup.2468. PMID 26216554.
- Parrott AC (March 2006). "Nicotine psychobiology: how chronic-dose prospective studies can illuminate some of the theoretical issues from acute-dose research". Psychopharmacology. 184 (3–4): 567–76. doi:10.1007/s00213-005-0294-y. PMID 16463194.
- ^ Parrott, Andrew C (April 2003). "Cigarette-Derived Nicotine is not a Medicine". The World Journal of Biological Psychiatry. 4 (2): 49–55. doi:10.3109/15622970309167951. ISSN 1562-2975.
Regular smokers need nicotine to remain feeling normal, and suffer from adverse moods without it... Smoking only generates mood changes in nicotine-deprived smokers, but these only represent the restoration of normal moods. When non-deprived smokers have a cigarette, their mood ratings remain unaltered... When smokers completed a brief mood self-rating for every cigarette over the day, normal moods were reported immediately after smoking, moods deteriorated in between cigarettes, and were normalized by the next cigarette (Parrott 1994)... Thus smoke inhalation in an abstinent smoker restores 'pleasure' to normal levels
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Franck C, Filion KB, Kimmelman J, Grad R, Eisenberg MJ (May 2016). "Ethical considerations of e-cigarette use for tobacco harm reduction". Respiratory Research. 17 (1): 53. doi:10.1186/s12931-016-0370-3. PMC 4869264. PMID 27184265.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ de Andrade, Marisa; Hastings, Gerald. "Tobacco Harm Reduction and Nicotine Containing Products" (PDF). Cancer Research UK. Cancer Research UK. p. 8. Retrieved 10 March 2016.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Electronic Cigarettes: Vulnerability of Youth". Pediatr Allergy Immunol Pulmonol. 28 (1): 2–6. 2015. doi:10.1089/ped.2015.0490. PMC 4359356. PMID 25830075.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Schraufnagel DE, Blasi F, Drummond MB, Lam DC, Latif E, Rosen MJ, Sansores R, Van Zyl-Smit R (September 2014). "Electronic cigarettes. A position statement of the forum of international respiratory societies". American Journal of Respiratory and Critical Care Medicine. 190 (6): 611–8. doi:10.1164/rccm.201407-1198PP. PMID 25006874.
- ^ National Center for Chronic Disease Prevention Health Promotion (US) Office on Smoking Health (2014). "The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, Chapter 5 – Nicotine" (PDF). Surgeon General of the United States: 107–138. PMID 24455788.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Jerry JM, Collins GB, Streem D (August 2015). "E-cigarettes: Safe to recommend to patients?". Cleveland Clinic Journal of Medicine. 82 (8): 521–6. doi:10.3949/ccjm.82a.14054. PMID 26270431.
Nicotine plays a direct role in carcinogenesis through a variety of mechanisms, including increasing the activity of tumor growth-promoting transcription factors, decreasing apoptosis, and increasing angiogenesis in tumors. Additionally, specific types of nicotinic acetylcholine receptors— eg, alpha 7 receptors, which are stimulated by nicotine—are found in many malignant tumors and are thought to play a role in tumor progression.12 Blockade of alpha 7 nicotinic acetylcholine receptors has been shown to decrease the growth of certain cancers. However, these findings were from in vitro studies, and the concerns they raised have not been reflected in in vivo studies. Despite having been on the market for 30 years, nicotine replacement therapy has as yet not been associated with any "real world" increase in cancer risk.
- ^ Brandon TH, Goniewicz ML, Hanna NH, Hatsukami DK, Herbst RS, Hobin JA, Ostroff JS, Shields PG, Toll BA, Tyne CA, Viswanath K, Warren GW (February 2015). "Electronic nicotine delivery systems: a policy statement from the American Association for Cancer Research and the American Society of Clinical Oncology". Clinical Cancer Research. 21 (3): 514–25. doi:10.1158/1078-0432.CCR-14-2544. PMID 25573384.
- ^ McNeill A, Brose LS, Calder R, Hitchman SC, Hajek P, McRobbie H (August 2015). "E-cigarettes: an evidence update" (PDF). UK: Public Health England. p. 63.
- ^ Stead LF, Perera R, Bullen C, Mant D, Lancaster T (January 2008). Stead LF (ed.). "Nicotine replacement therapy for smoking cessation". The Cochrane Database of Systematic Reviews (1): CD000146. doi:10.1002/14651858.CD000146.pub3. PMID 18253970.
- Pierce JP, Cummins SE, White MM, Humphrey A, Messer K (April 2012). "Nicotine Replacement for Smoking Cessation: Do We Need to Change Policy?". Annual Review of Public Health. 33: 341–56. doi:10.1146/annurev-publhealth-031811-124624. PMID 22224888.
- Hughes, J. R. (1989). "Dependence potential and abuse liability of nicotine replacement therapies". Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 43 (1): 11–17. ISSN 0753-3322. PMID 2659095.
- ^ Tomizawa M, Casida JE (2005). "Neonicotinoid insecticide toxicology: mechanisms of selective action" (PDF). Annual Review of Pharmacology and Toxicology. 45: 247–68. doi:10.1146/annurev.pharmtox.45.120403.095930. PMID 15822177.
- "Tobacco and its evil cousin nicotine are good as a pesticide – American Chemical Society". American Chemical Society. Retrieved 29 October 2018.
- ^ USEPA (3 June 2009). "Nicotine; Product Cancellation Order". Federal Register: 26695–26696. Retrieved 8 April 2012.
- ^ US Code of Federal Regulations. 7 CFR 205.602 – Nonsynthetic substances prohibited for use in organic crop production
- Tharp, Cecil (5 September 2014). "Safety for Homemade Remedies for Pest Control" (PDF). Montana Pesticide Bulletin. Montana State University. Retrieved 29 October 2018.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Michalski B, Herrmann M, Solecki R (July 2017). "". Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz (in German). 60 (7): 768–773. doi:10.1007/s00103-017-2556-3. PMID 28508955.
- European Food Safety Authority (7 May 2009). "Potential risks for public health due to the presence of nicotine in wild mushrooms". EFSA Journal. 7 (5): 286r. doi:10.2903/j.efsa.2009.286r.
- Abreu-Villaça Y, Levin ED (February 2017). "Developmental neurotoxicity of succeeding generations of insecticides". Environment International. 99: 55–77. doi:10.1016/j.envint.2016.11.019. PMC 5285268. PMID 27908457.
- Rodgman, Alan; Perfetti, Thomas A. (2009). The chemical components of tobacco and tobacco smoke. Boca Raton, FL: CRC Press. ISBN 978-1-4200-7883-1. LCCN 2008018913.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Heishman SJ, Kleykamp BA, Singleton EG (July 2010). "Meta-analysis of the acute effects of nicotine and smoking on human performance". Psychopharmacology. 210 (4): 453–69. doi:10.1007/s00213-010-1848-1. PMC 3151730. PMID 20414766.
- Sarter M (August 2015). "Behavioral-Cognitive Targets for Cholinergic Enhancement". Current Opinion in Behavioral Sciences. 4: 22–26. doi:10.1016/j.cobeha.2015.01.004. PMC 5466806. PMID 28607947.
- "Nicotine: Biological activity". IUPHAR/BPS Guide to Pharmacology. International Union of Basic and Clinical Pharmacology. Retrieved 7 February 2016.
Kis as follows; α2β4=9900nM , α3β2=14nM , α3β4=187nM , α4β2=1nM . Due to the heterogeneity of nACh channels we have not tagged a primary drug target for nicotine, although the α4β2 is reported to be the predominant high affinity subtype in the brain which mediates nicotine addiction
- Majdi A, Kamari F, Vafaee MS, Sadigh-Eteghad S (October 2017). "Revisiting nicotine's role in the ageing brain and cognitive impairment". Reviews in the Neurosciences. 28 (7): 767–781. doi:10.1515/revneuro-2017-0008. PMID 28586306.
- Uban KA, Horton MK, Jacobus J, Heyser C, Thompson WK, Tapert SF, Madden PA, Sowell ER (August 2018). "Biospecimens and the ABCD study: Rationale, methods of collection, measurement and early data". Developmental Cognitive Neuroscience. 32: 97–106. doi:10.1016/j.dcn.2018.03.005. PMID 29606560.
- Siqueira LM (January 2017). "Nicotine and Tobacco as Substances of Abuse in Children and Adolescents". Pediatrics. 139 (1): e20163436. doi:10.1542/peds.2016-3436. PMID 27994114.
- ^ Stolerman IP, Jarvis MJ (January 1995). "The scientific case that nicotine is addictive". Psychopharmacology. 117 (1): 2–10, discussion 14–20. doi:10.1007/BF02245088. PMID 7724697.
- ^ Wilder, Natalie; Daley, Claire; Sugarman, Jane; Partridge, James (April 2016). "Nicotine without smoke: Tobacco harm reduction". UK: Royal College of Physicians. pp. 58, 125.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ El Sayed KA, Sylvester PW (June 2007). "Biocatalytic and semisynthetic studies of the anticancer tobacco cembranoids". Expert Opinion on Investigational Drugs. 16 (6): 877–87. doi:10.1517/13543784.16.6.877. PMID 17501699.
- Rahman MA, Hann N, Wilson A, Worrall-Carter L (2014). "Electronic cigarettes: patterns of use, health effects, use in smoking cessation and regulatory issues". Tobacco Induced Diseases. 12 (1): 21. doi:10.1186/1617-9625-12-21. PMC 4350653. PMID 25745382.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Morjaria JB, Mondati E, Polosa R (2017). "E-cigarettes in patients with COPD: current perspectives". International Journal of Chronic Obstructive Pulmonary Disease. 12: 3203–3210. doi:10.2147/COPD.S135323. PMC 5677304. PMID 29138548.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Mishra A, Chaturvedi P, Datta S, Sinukumar S, Joshi P, Garg A (2015). "Harmful effects of nicotine". Indian Journal of Medical and Paediatric Oncology. 36 (1): 24–31. doi:10.4103/0971-5851.151771. PMC 4363846. PMID 25810571.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - "Gender, women, and the tobacco epidemic" (PDF). World Health Organization. 2010.
- Golub JS, Samy RN (October 2015). "Preventing or reducing smoking-related complications in otologic and neurotologic surgery". Current Opinion in Otolaryngology & Head and Neck Surgery. 23 (5): 334–40. doi:10.1097/MOO.0000000000000184. PMID 26339963.
- Dupont P, Benyamina A, Aubin HJ (December 2016). "". Revue des Maladies Respiratoires. 33 (10): 892–898. doi:10.1016/j.rmr.2016.05.002. PMID 27242286.
- Dinakar C, O'Connor GT (October 2016). "The Health Effects of Electronic Cigarettes". The New England Journal of Medicine. 375 (14): 1372–1381. doi:10.1056/NEJMra1502466. PMID 27705269.
- ^ Bhatnagar A, Whitsel LP, Ribisl KM, Bullen C, Chaloupka F, Piano MR, Robertson RM, McAuley T, Goff D, Benowitz N (October 2014). "Electronic cigarettes: a policy statement from the American Heart Association". Circulation. 130 (16): 1418–36. doi:10.1161/CIR.0000000000000107. PMID 25156991.
- ^ Lee PN (December 2013). "Epidemiological evidence relating snus to health—an updated review based on recent publications". Harm Reduction Journal. 10 (1): 36. doi:10.1186/1477-7517-10-36. PMC 4029226. PMID 24314326.
{{cite journal}}: CS1 maint: unflagged free DOI (link) This article incorporates text by Peter N Lee available under the CC BY 2.0 license.
This article incorporates text by Peter N Lee available under the CC BY 2.0 license.
- Nicotine without smoke: Tobacco harm reduction. London: Royal College of Physicians. 2016. p. 151. ISBN 978-1-86016-600-6.
- Lødrup Carlsen KC, Skjerven HO, Carlsen KH (February 2018). "The toxicity of E-cigarettes and children's respiratory health". Paediatric Respiratory Reviews. 28: 63–67. doi:10.1016/j.prrv.2018.01.002. PMID 29580719.
- ^ Fujii T, Mashimo M, Moriwaki Y, Misawa H, Ono S, Horiguchi K, Kawashima K (2017). "Expression and Function of the Cholinergic System in Immune Cells". Frontiers in Immunology. 8: 1085. doi:10.3389/fimmu.2017.01085. PMC 5592202. PMID 28932225.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Bai X, Stitzel JA, Bai A, Zambrano CA, Phillips M, Marrack P, Chan ED (September 2017). "Nicotine Impairs Macrophage Control of Mycobacterium tuberculosis". American Journal of Respiratory Cell and Molecular Biology. 57 (3): 324–333. doi:10.1165/rcmb.2016-0270OC. PMC 5625222. PMID 28398760.
- Orsini, Jean-Claude (June 2001). "Dépendance tabagique et contrôle central de la glycémie et de l'appétit" [Dependence on tobacco smoking and brain systems controlling glycemia and appetite]. Alcoologie et Addictologie (in French). 23 (2 Suppl): 28S–36S. ISSN 1620-4522. INIST 1081638.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Chen H, Vlahos R, Bozinovski S, Jones J, Anderson GP, Morris MJ (April 2005). "Effect of Short-Term Cigarette Smoke Exposure on Body Weight, Appetite and Brain Neuropeptide Y in Mice". Neuropsychopharmacology. 30 (4): 713–9. doi:10.1038/sj.npp.1300597. PMID 15508020.
{{cite journal}}: Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help) - ^ Audrain-McGovern J, Benowitz NL (July 2011). "Cigarette smoking, nicotine, and body weight". Clinical Pharmacology and Therapeutics. 90 (1): 164–8. doi:10.1038/clpt.2011.105. PMC 3195407. PMID 21633341.
- ^ Chiolero A, Faeh D, Paccaud F, Cornuz J (April 2008). "Consequences of smoking for body weight, body fat distribution, and insulin resistance". The American Journal of Clinical Nutrition. 87 (4): 801–9. doi:10.1093/ajcn/87.4.801. PMID 18400700.
- ^ Farsalinos KE, Polosa R (April 2014). "Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review". Therapeutic Advances in Drug Safety. 5 (2): 67–86. doi:10.1177/2042098614524430. PMC 4110871. PMID 25083263.
- ^ Hua M, Talbot P (December 2016). "Potential health effects of electronic cigarettes: A systematic review of case reports". Preventive Medicine Reports. 4: 169–78. doi:10.1016/j.pmedr.2016.06.002. PMC 4929082. PMID 27413679.
 This article incorporates text by My Hua and Prue Talbot available under the CC BY 4.0 license.
This article incorporates text by My Hua and Prue Talbot available under the CC BY 4.0 license.
- ^ Benowitz NL, Burbank AD (August 2016). "Cardiovascular toxicity of nicotine: Implications for electronic cigarette use". Trends in Cardiovascular Medicine. 26 (6): 515–23. doi:10.1016/j.tcm.2016.03.001. PMC 4958544. PMID 27079891.
- Morris PB, Ference BA, Jahangir E, Feldman DN, Ryan JJ, Bahrami H, El-Chami MF, Bhakta S, Winchester DE, Al-Mallah MH, Sanchez Shields M, Deedwania P, Mehta LS, Phan BA, Benowitz NL (September 2015). "Cardiovascular Effects of Exposure to Cigarette Smoke and Electronic Cigarettes: Clinical Perspectives From the Prevention of Cardiovascular Disease Section Leadership Council and Early Career Councils of the American College of Cardiology". Journal of the American College of Cardiology. 66 (12): 1378–91. doi:10.1016/j.jacc.2015.07.037. PMID 26383726.
- ^ Stead LF, Perera R, Bullen C, Mant D, Hartmann-Boyce J, Cahill K, Lancaster T (November 2012). "Nicotine replacement therapy for smoking cessation". The Cochrane Database of Systematic Reviews. 11: CD000146. doi:10.1002/14651858.CD000146.pub4. PMID 23152200.
- ^ Hansson J, Galanti MR, Hergens MP, Fredlund P, Ahlbom A, Alfredsson L, Bellocco R, Eriksson M, Hallqvist J, Hedblad B, Jansson JH, Nilsson P, Pedersen N, Trolle Lagerros Y, Ostergren PO, Magnusson C (October 2012). "Use of snus and acute myocardial infarction: pooled analysis of eight prospective observational studies". European Journal of Epidemiology. 27 (10): 771–9. doi:10.1007/s10654-012-9704-8. PMID 22722951.
- "Nicotine". National Institute on Drug Abuse. June 2007.
- "WHO Right to Call for E-Cigarette Regulation". World Lung Federation. 26 August 2014.
- Zhang S, Day I, Ye S (February 2001). "Nicotine induced changes in gene expression by human coronary artery endothelial cells". Atherosclerosis. 154 (2): 277–83. doi:10.1016/S0021-9150(00)00475-5. PMID 11166759.
- Hawkins BT, Brown RC, Davis TP (February 2002). "Smoking and ischemic stroke: a role for nicotine?". Trends in Pharmacological Sciences. 23 (2): 78–82. doi:10.1016/S0165-6147(02)01893-X. PMID 11830264.
- Jandíková H, Dušková M, Stárka L (September 2017). "The influence of smoking and cessation on the human reproductive hormonal balance" (PDF). Physiological Research. 66 (Supplementum 3): S323–S331. PMID 28948816.
- Detailed reference list is located on a separate image page.
- Cardinale A, Nastrucci C, Cesario A, Russo P (January 2012). "Nicotine: specific role in angiogenesis, proliferation and apoptosis". Critical Reviews in Toxicology. 42 (1): 68–89. doi:10.3109/10408444.2011.623150. PMID 22050423.
- ^ Sanner T, Grimsrud TK (2015). "Nicotine: Carcinogenicity and Effects on Response to Cancer Treatment – A Review". Frontiers in Oncology. 5: 196. doi:10.3389/fonc.2015.00196. PMC 4553893. PMID 26380225.
{{cite journal}}: CS1 maint: unflagged free DOI (link) This article incorporates text by Tore Sanner and Tom K. Grimsrud available under the CC BY 4.0 license.
This article incorporates text by Tore Sanner and Tom K. Grimsrud available under the CC BY 4.0 license.
- Hecht SS (July 1999). "Tobacco smoke carcinogens and lung cancer". Journal of the National Cancer Institute. 91 (14): 1194–210. doi:10.1093/jnci/91.14.1194. PMID 10413421.
- Wu WK, Cho CH (April 2004). "The pharmacological actions of nicotine on the gastrointestinal tract". Journal of Pharmacological Sciences. 94 (4): 348–58. doi:10.1254/jphs.94.348. PMID 15107574.
- Chowdhury P, Udupa KB (December 2006). "Nicotine as a mitogenic stimulus for pancreatic acinar cell proliferation". World Journal of Gastroenterology. 12 (46): 7428–32. doi:10.3748/wjg.v12.i46.7428. PMC 4087586. PMID 17167829.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Wong HP, Yu L, Lam EK, Tai EK, Wu WK, Cho CH (June 2007). "Nicotine promotes colon tumor growth and angiogenesis through beta-adrenergic activation". Toxicological Sciences. 97 (2): 279–87. doi:10.1093/toxsci/kfm060. PMID 17369603.
- Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M (October 2003). "Nicotine enhances neovascularization and promotes tumor growth". Molecules and Cells. 16 (2): 143–6. PMID 14651253.
- Ye YN, Liu ES, Shin VY, Wu WK, Luo JC, Cho CH (January 2004). "Nicotine promoted colon cancer growth via epidermal growth factor receptor, c-Src, and 5-lipoxygenase-mediated signal pathway". The Journal of Pharmacology and Experimental Therapeutics. 308 (1): 66–72. doi:10.1124/jpet.103.058321. PMID 14569062.
- Davis R, Rizwani W, Banerjee S, Kovacs M, Haura E, Coppola D, Chellappan S (October 2009). Pao W (ed.). "Nicotine promotes tumor growth and metastasis in mouse models of lung cancer". PLOS One. 4 (10): e7524. Bibcode:2009PLoSO...4.7524D. doi:10.1371/journal.pone.0007524. PMC 2759510. PMID 19841737.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Knezevich A, Muzic J, Hatsukami DK, Hecht SS, Stepanov I (February 2013). "Nornicotine nitrosation in saliva and its relation to endogenous synthesis of N'-nitrosonornicotine in humans". Nicotine & Tobacco Research. 15 (2): 591–5. doi:10.1093/ntr/nts172. PMC 3611998. PMID 22923602.
- Kothari AN, Mi Z, Zapf M, Kuo PC (2014). "Novel clinical therapeutics targeting the epithelial to mesenchymal transition". Clinical and Translational Medicine. 3: 35. doi:10.1186/s40169-014-0035-0. PMC 4198571. PMID 25343018.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Dugas EN, Sylvestre MP, O'Loughlin EK, Brunet J, Kakinami L, Constantin E, O'Loughlin J (February 2017). "Nicotine dependence and sleep quality in young adults". Addictive Behaviors. 65: 154–160. doi:10.1016/j.addbeh.2016.10.020. PMID 27816041.
- Cohrs S, Rodenbeck A, Riemann D, Szagun B, Jaehne A, Brinkmeyer J, Gründer G, Wienker T, Diaz-Lacava A, Mobascher A, Dahmen N, Thuerauf N, Kornhuber J, Kiefer F, Gallinat J, Wagner M, Kunz D, Grittner U, Winterer G (May 2014). "Impaired sleep quality and sleep duration in smokers-results from the German Multicenter Study on Nicotine Dependence". Addiction Biology. 19 (3): 486–96. doi:10.1111/j.1369-1600.2012.00487.x. PMID 22913370.
- Jaehne A, Unbehaun T, Feige B, Herr S, Appel A, Riemann D (March 2014). "The influence of 8 and 16 mg nicotine patches on sleep in healthy non-smokers". Pharmacopsychiatry. 47 (2): 73–8. doi:10.1055/s-0034-1371867. PMID 24687640.
- Yang Y, Paspalas CD, Jin LE, Picciotto MR, Arnsten AF, Wang M (July 2013). "Nicotinic α7 receptors enhance NMDA cognitive circuits in dorsolateral prefrontal cortex". Proceedings of the National Academy of Sciences of the United States of America. 110 (29): 12078–83. Bibcode:2013PNAS..11012078Y. doi:10.1073/pnas.1307849110. PMC 3718126. PMID 23818597.
- Madrid-López N, Estrada J, Díaz J, Bassi A, Délano PH, Ocampo-Garcés A (2017). "The Sleep-Wake Cycle in the Nicotinic Alpha-9 Acetylcholine Receptor Subunit Knock-Out Mice". Frontiers in Cellular Neuroscience. 11: 302. doi:10.3389/fncel.2017.00302. PMC 5641320. PMID 29066952.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Alawsi F, Nour R, Prabhu S (August 2015). "Are e-cigarettes a gateway to smoking or a pathway to quitting?". British Dental Journal. 219 (3): 111–5. doi:10.1038/sj.bdj.2015.591. PMID 26271862.
- "State Health Officer's Report on E-Cigarettes: A Community Health Threat" (PDF). California Tobacco Control Program. California Department of Public Health. January 2015.
- "Electronic Cigarettes – An Overview" (PDF). German Cancer Research Center. 2013.
- ^ Bruin JE, Gerstein HC, Holloway AC (August 2010). "Long-term consequences of fetal and neonatal nicotine exposure: a critical review". Toxicological Sciences. 116 (2): 364–74. doi:10.1093/toxsci/kfq103. PMC 2905398. PMID 20363831.
- England LJ, Kim SY, Tomar SL, Ray CS, Gupta PC, Eissenberg T, Cnattingius S, Bernert JT, Tita AT, Winn DM, Djordjevic MV, Lambe M, Stamilio D, Chipato T, Tolosa JE (31 December 2010). "Non-cigarette tobacco use among women and adverse pregnancy outcomes". Acta Obstetricia et Gynecologica Scandinavica. 89 (4): 454–64. doi:10.3109/00016341003605719. PMC 5881107. PMID 20225987.
- "The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, Chapter 9 – Reproductive Outcomes" (PDF). Surgeon General of the United States. 2014: 107–138. PMID 24455788.
{{cite journal}}: Cite journal requires|journal=(help) - Behnke M, Smith VC (March 2013). "Prenatal substance abuse: short- and long-term effects on the exposed fetus". Pediatrics. 131 (3): e1009–24. doi:10.1542/peds.2012-3931. PMID 23439891.
- "Chemicals Known To The State To Cause Cancer Or Reproductive Toxicity" (PDF). Safe Drinking Water And Toxic Enforcement Act Of 1986. State Of California Environmental Protection Agency, Office of Environmental Health Hazard Assessment. 18 December 2009. Archived from the original (PDF) on 28 January 2010.
{{cite web}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help) - Kandel DB, ed. (2002). Stages and Pathways of Drug Involvement: Examining the Gateway Hypothesis. Cambridge University Press. pp. 3–10. ISBN 978-0-521-78969-1.
- Kandel ER, Kandel DB (September 2014). "Shattuck Lecture. A molecular basis for nicotine as a gateway drug". The New England Journal of Medicine. 371 (10): 932–43. doi:10.1056/NEJMsa1405092. PMC 4353486. PMID 25184865.
- Keyes KM, Hamilton A, Kandel DB (June 2016). "Birth Cohorts Analysis of Adolescent Cigarette Smoking and Subsequent Marijuana and Cocaine Use". American Journal of Public Health. 106 (6): 1143–9. doi:10.2105/AJPH.2016.303128. PMC 4880234. PMID 27077359.
- Panlilio LV, Zanettini C, Barnes C, Solinas M, Goldberg SR (June 2013). "Prior exposure to THC increases the addictive effects of nicotine in rats". Neuropsychopharmacology. 38 (7): 1198–208. doi:10.1038/npp.2013.16. PMC 3656362. PMID 23314220.
- Kirby T, Barry AE (August 2012). "Alcohol as a gateway drug: a study of US 12th graders" (PDF). The Journal of School Health. 82 (8): 371–9. doi:10.1111/j.1746-1561.2012.00712.x. PMID 22712674.
- Okamoto M, Kita T, Okuda H, Tanaka T, Nakashima T (July 1994). "Effects of aging on acute toxicity of nicotine in rats". Pharmacology & Toxicology. 75 (1): 1–6. doi:10.1111/j.1600-0773.1994.tb00316.x. PMID 7971729.
- Toxicology and Applied Pharmacology. Vol. 44, Pg. 1, 1978.
- ^ Schep LJ, Slaughter RJ, Beasley DM (September 2009). "Nicotinic plant poisoning". Clinical Toxicology. 47 (8): 771–81. doi:10.1080/15563650903252186. PMID 19778187.
- Smolinske SC, Spoerke DG, Spiller SK, Wruk KM, Kulig K, Rumack BH (January 1988). "Cigarette and nicotine chewing gum toxicity in children". Human Toxicology. 7 (1): 27–31. doi:10.1177/096032718800700105. PMID 3346035.
- Furer V, Hersch M, Silvetzki N, Breuer GS, Zevin S (March 2011). "Nicotiana glauca (tree tobacco) intoxication--two cases in one family". Journal of Medical Toxicology. 7 (1): 47–51. doi:10.1007/s13181-010-0102-x. PMC 3614112. PMID 20652661.
- Gehlbach SH, Williams WA, Perry LD, Woodall JS (September 1974). "Green-tobacco sickness. An illness of tobacco harvesters". JAMA. 229 (14): 1880–3. doi:10.1001/jama.1974.03230520022024. PMID 4479133.
- "CDC – NIOSH Pocket Guide to Chemical Hazards – Nicotine". www.cdc.gov. Retrieved 20 November 2015.
- "Consumer Updates: Nicotine Replacement Therapy Labels May Change". FDA. 1 April 2013.
- "Do You Vape? See These Tips on How to Keep E-Liquids Away from Children". United States Department of Health and Human Services. United States Food and Drug Administration. 2 May 2018.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- Das S, Prochaska JJ (October 2017). "Innovative approaches to support smoking cessation for individuals with mental illness and co-occurring substance use disorders". Expert Review of Respiratory Medicine. 11 (10): 841–850. doi:10.1080/17476348.2017.1361823. PMC 5790168. PMID 28756728.
- Heishman SJ, Kleykamp BA, Singleton EG (July 2010). "Meta-analysis of the acute effects of nicotine and smoking on human performance". Psychopharmacology. 210 (4): 453–69. doi:10.1007/s00213-010-1848-1. PMC 3151730. PMID 20414766.
The significant effects of nicotine on motor abilities, attention, and memory likely represent true performance enhancement because they are not confounded by withdrawal relief. The beneficial cognitive effects of nicotine have implications for initiation of smoking and maintenance of tobacco dependence.
- Baraona LK, Lovelace D, Daniels JL, McDaniel L (May 2017). "Tobacco Harms, Nicotine Pharmacology, and Pharmacologic Tobacco Cessation Interventions for Women". Journal of Midwifery & Women's Health. 62 (3): 253–269. doi:10.1111/jmwh.12616. PMID 28556464.
- ^ Parrott, Andrew C (April 2003). "Cigarette-Derived Nicotine is not a Medicine". The World Journal of Biological Psychiatry. 4 (2): 49–55. doi:10.3109/15622970309167951. ISSN 1562-2975.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Bruijnzeel AW (May 2012). "Tobacco addiction and the dysregulation of brain stress systems". Neuroscience and Biobehavioral Reviews. 36 (5): 1418–41. doi:10.1016/j.neubiorev.2012.02.015. PMC 3340450. PMID 22405889.
Discontinuation of smoking leads to negative affective symptoms such as depressed mood, increased anxiety, and impaired memory and attention...Smoking cessation leads to a relatively mild somatic withdrawal syndrome and a severe affective withdrawal syndrome that is characterized by a decrease in positive affect, an increase in negative affect, craving for tobacco, irritability, anxiety, difficulty concentrating, hyperphagia, restlessness, and a disruption of sleep. Smoking during the acute withdrawal phase reduces craving for cigarettes and returns cognitive abilities to pre-smoking cessation level
- ^ Nestler EJ (December 2013). "Cellular basis of memory for addiction". Dialogues in Clinical Neuroscience. 15 (4): 431–43. PMC 3898681. PMID 24459410.
- ^ Ruffle JK (November 2014). "Molecular neurobiology of addiction: what's all the (Δ)FosB about?". The American Journal of Drug and Alcohol Abuse. 40 (6): 428–37. doi:10.3109/00952990.2014.933840. PMID 25083822.
The knowledge of ΔFosB induction in chronic drug exposure provides a novel method for the evaluation of substance addiction profiles (i.e. how addictive they are). Xiong et al. used this premise to evaluate the potential addictive profile of propofol (119). Propofol is a general anaesthetic, however its abuse for recreational purpose has been documented (120). Using control drugs implicated in both ΔFosB induction and addiction (ethanol and nicotine), ...
Conclusions
ΔFosB is an essential transcription factor implicated in the molecular and behavioral pathways of addiction following repeated drug exposure. The formation of ΔFosB in multiple brain regions, and the molecular pathway leading to the formation of AP-1 complexes is well understood. The establishment of a functional purpose for ΔFosB has allowed further determination as to some of the key aspects of its molecular cascades, involving effectors such as GluR2 (87,88), Cdk5 (93) and NFkB (100). Moreover, many of these molecular changes identified are now directly linked to the structural, physiological and behavioral changes observed following chronic drug exposure (60,95,97,102). New frontiers of research investigating the molecular roles of ΔFosB have been opened by epigenetic studies, and recent advances have illustrated the role of ΔFosB acting on DNA and histones, truly as a molecular switch (34). As a consequence of our improved understanding of ΔFosB in addiction, it is possible to evaluate the addictive potential of current medications (119), as well as use it as a biomarker for assessing the efficacy of therapeutic interventions (121,122,124). - Marttila K, Raattamaa H, Ahtee L (July 2006). "Effects of chronic nicotine administration and its withdrawal on striatal FosB/DeltaFosB and c-Fos expression in rats and mice". Neuropharmacology. 51 (1): 44–51. doi:10.1016/j.neuropharm.2006.02.014. PMID 16631212.
- "Effective Clinical Tobacco Intervention". Therapeutics Letter (21): 1–4. September–October 1997.
- Lagrue, Gilbert; Cormier, Anne (June 2001). "Des récepteurs nicotiniques à la dépendance tabagique: Perspectives thérapeutiques" [From nicotinic receptors to smoking dependence: Therapeutic prospects]. Alcoologie et Addictologie (in French). 23 (2): 39S–42S. INIST 1081618.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Pomerleau OF, Pomerleau CS (1984). "Neuroregulators and the reinforcement of smoking: towards a biobehavioral explanation". Neuroscience and Biobehavioral Reviews. 8 (4): 503–13. doi:10.1016/0149-7634(84)90007-1. PMID 6151160.
- Pomerleau OF, Rosecrans J (1989). "Neuroregulatory effects of nicotine". Psychoneuroendocrinology. 14 (6): 407–23. doi:10.1016/0306-4530(89)90040-1. PMID 2560221.
- Kenny PJ, Markou A (June 2006). "Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity". Neuropsychopharmacology. 31 (6): 1203–11. doi:10.1038/sj.npp.1300905. PMID 16192981.
- Golding JF, Mangan GL (1989). "Factors Governing Recruitment to and Maintenance of Smoking". In Einstein S (ed.). Drug and Alcohol Use. pp. 101–17. doi:10.1007/978-1-4899-0888-9_9. ISBN 978-1-4899-0890-2.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help); Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Nesbitt P (1969). Smoking, physiological arousal, and emotional response. Unpublished doctoral dissertation, Columbia University.
- Parrott AC (January 1998). "Nesbitt's Paradox resolved? Stress and arousal modulation during cigarette smoking" (PDF). Addiction. 93 (1): 27–39. CiteSeerX 10.1.1.465.2496. doi:10.1046/j.1360-0443.1998.931274.x. PMID 9624709.
- Wadgave U, Nagesh L (July 2016). "Nicotine Replacement Therapy: An Overview". International Journal of Health Sciences. 10 (3): 425–35. PMC 5003586. PMID 27610066.
- Katzung, Bertram G. (2006). Basic and Clinical Pharmacology. New York: McGraw-Hill Medical. pp. 99–105.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Xiu X, Puskar NL, Shanata JA, Lester HA, Dougherty DA (March 2009). "Nicotine binding to brain receptors requires a strong cation-pi interaction". Nature. 458 (7237): 534–7. Bibcode:2009Natur.458..534X. doi:10.1038/nature07768. PMC 2755585. PMID 19252481.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 369, 372–373. ISBN 9780071481274.
- ^ Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E (June 2011). "The role of the central ghrelin system in reward from food and chemical drugs". Molecular and Cellular Endocrinology. 340 (1): 80–7. doi:10.1016/j.mce.2011.02.017. hdl:2077/26318. PMID 21354264.
This reward link comprises a dopamine projection from the ventral tegmental area (VTA) to the nucleus accumbens together with a cholinergic input, arising primarily from the laterodorsal tegmental area.
- Levine A, Huang Y, Drisaldi B, Griffin EA, Pollak DD, Xu S, Yin D, Schaffran C, Kandel DB, Kandel ER (November 2011). "Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine". Science Translational Medicine. 3 (107): 107ra109. doi:10.1126/scitranslmed.3003062. PMC 4042673. PMID 22049069.
- Volkow ND (November 2011). "Epigenetics of nicotine: another nail in the coughing". Science Translational Medicine. 3 (107): 107ps43. doi:10.1126/scitranslmed.3003278. PMC 3492949. PMID 22049068.
- Yoshida T, Sakane N, Umekawa T, Kondo M (January 1994). "Effect of nicotine on sympathetic nervous system activity of mice subjected to immobilization stress". Physiology & Behavior. 55 (1): 53–7. doi:10.1016/0031-9384(94)90009-4. PMID 8140174.
- Marieb, Elaine N.; Hoehn, Katja (2007). Human Anatomy & Physiology (7th Ed.). Pearson. pp. ?. ISBN 978-0-8053-5909-1.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Henningfield, Jack E.; Calvento, Emma; Pogun, Sakire (2009). Nicotine Psychopharmacology. Springer. pp. 35, 37. ISBN 978-3-540-69248-5.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Le Houezec J (September 2003). "Role of nicotine pharmacokinetics in nicotine addiction and nicotine replacement therapy: a review". The International Journal of Tuberculosis and Lung Disease. 7 (9): 811–9. PMID 12971663.
- Benowitz NL, Jacob P, Jones RT, Rosenberg J (May 1982). "Interindividual variability in the metabolism and cardiovascular effects of nicotine in man". The Journal of Pharmacology and Experimental Therapeutics. 221 (2): 368–72. PMID 7077531.
- Russell MA, Jarvis M, Iyer R, Feyerabend C. Relation of nicotine yield of cigarettes to blood nicotine concentrations in smokers. Br Med J. 1980 April 5; 280(6219): 972–976.
- Bhalala, Oneil (Spring 2003). "Detection of Cotinine in Blood Plasma by HPLC MS/MS". MIT Undergraduate Research Journal. 8: 45–50. Archived from the original on 24 December 2013.
{{cite journal}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help); Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Hukkanen J, Jacob P, Benowitz NL (March 2005). "Metabolism and disposition kinetics of nicotine". Pharmacological Reviews. 57 (1): 79–115. doi:10.1124/pr.57.1.3. PMID 15734728.
- Petrick LM, Svidovsky A, Dubowski Y (January 2011). "Thirdhand smoke: heterogeneous oxidation of nicotine and secondary aerosol formation in the indoor environment". Environmental Science & Technology. 45 (1): 328–33. Bibcode:2011EnST...45..328P. doi:10.1021/es102060v. PMID 21141815.
{{cite journal}}: Unknown parameter|lay-source=ignored (help); Unknown parameter|lay-summary=ignored (help) - Benowitz NL, Herrera B, Jacob P (September 2004). "Mentholated cigarette smoking inhibits nicotine metabolism". The Journal of Pharmacology and Experimental Therapeutics. 310 (3): 1208–15. doi:10.1124/jpet.104.066902. PMID 15084646.
- "NFPA Hazard Rating Information for Common Chemicals". Archived from the original on 17 February 2015. Retrieved 15 March 2015.
{{cite web}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help) - "L-Nicotine Material Safety Data Sheet". Sciencelab.com, Inc.
- Gause GF (1941). "Chapter V: Analysis of various biological processes by the study of the differential action of optical isomers". In Luyet, B. J. (ed.). Optical Activity and Living Matter. A series of monographs on general physiology. Vol. 2. Normandy, Missouri: Biodynamica.
- ^ Henry, Thomas Anderson (1949). The Plant Alkaloids (PDF) (4th ed.). Philadelphia, Toronto: The Blakiston Company.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - "Tobacco (leaf tobacco)". Transportation Information Service.
- Domino EF, Hornbach E, Demana T (August 1993). "The nicotine content of common vegetables". The New England Journal of Medicine. 329 (6): 437. doi:10.1056/NEJM199308053290619. PMID 8326992.
- Moldoveanu, Serban C.; Scott, Wayne A.; Lawson, Darlene M. (2016). "Nicotine Analysis in Several Non-Tobacco Plant Materials". Beiträge zur Tabakforschung International/Contributions to Tobacco Research. 27 (2): 54–59. doi:10.1515/cttr-2016-0008. ISSN 1612-9237.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Lamberts BL, Dewey LJ, Byerrum RU (May 1959). "Ornithine as a precursor for the pyrrolidine ring of nicotine". Biochimica et Biophysica Acta. 33 (1): 22–6. doi:10.1016/0006-3002(59)90492-5. PMID 13651178.
- Dawson RF, Christman DR, d'Adamo A, Solt ML, Wolf AP (1960). "The Biosynthesis of Nicotine from Isotopically Labeled Nicotinic Acids". Journal of the American Chemical Society. 82 (10): 2628–2633. doi:10.1021/ja01495a059.
- Ashihara, Hiroshi; Crozier, Alan; Komamine, Atsushi (eds.). Plant metabolism and biotechnology. Cambridge: Wiley. ISBN 978-0-470-74703-2.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Benowitz NL, Hukkanen J, Jacob P (1 January 2009). "Nicotine Chemistry, Metabolism, Kinetics and Biomarkers". Nicotine Psychopharmacology. Handbook of Experimental Pharmacology. Vol. 192. pp. 29–60. doi:10.1007/978-3-540-69248-5_2. ISBN 978-3-540-69246-1. PMC 2953858. PMID 19184645.
- Baselt, Randall Clint (2014). Disposition of Toxic Drugs and Chemicals in Man (10th ed.). Biomedical Publications. pp. 1452–6. ISBN 978-0-9626523-9-4.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Mündel T, Jones DA (July 2006). "Effect of transdermal nicotine administration on exercise endurance in men". Experimental Physiology. 91 (4): 705–13. doi:10.1113/expphysiol.2006.033373. PMID 16627574.
- ^ "FDA's Plan for Tobacco and Nicotine Regulation". United States Department of Health and Human Services. United States Food and Drug Administration. 15 March 2018.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Dale MM, Ritter JM, Fowler RJ, Rang HP. Rang & Dale's Pharmacology (6th ed.). Churchill Livingstone. p. 598. ISBN 978-0-8089-2354-1.
- Staff IFOAM. "Criticisms and Frequent Misconceptions about Organic Agriculture: The Counter-Arguments: Misconception". Archived from the original on 16 October 2013.
{{cite web}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help) - USEPA (29 October 2008). "Nicotine; Notice of Receipt of Request to Voluntarily Cancel a Pesticide Registration". Federal Register: 64320–64322. Retrieved 8 April 2012.
- Rodu B (July 2011). "The scientific foundation for tobacco harm reduction, 2006–2011". Harm Reduction Journal. 8 (1): 19. doi:10.1186/1477-7517-8-19. PMC 3161854. PMID 21801389.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Henningfield JE, Zeller M (March 2006). "Nicotine psychopharmacology research contributions to United States and global tobacco regulation: a look back and a look forward". Psychopharmacology. 184 (3–4): 286–91. doi:10.1007/s00213-006-0308-4. PMID 16463054.
- Posselt W, Reimann L (1828). "Chemische Untersuchung des Tabaks und Darstellung eines eigenthümlich wirksamen Prinzips dieser Pflanze" [Chemical investigation of tobacco and preparation of a characteristically active constituent of this plant]. Magazin für Pharmacie (in German). 6 (24): 138–161.
- Melsens, Louis-Henri-Frédéric (1843). "Note sur la nicotine" [Note on nicotine]. Annales de Chimie et de Physique. third series (in French). 9: 465–479, see especially page 470.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Pinner A, Wolffenstein R (1891). "Ueber Nicotin" [About nicotine]. Berichte der Deutschen Chemischen Gesellschaft (in German). 24: 1373–1377. doi:10.1002/cber.189102401242.
- Pinner A (1893). "Ueber Nicotin. Die Constitution des Alkaloïds" [About nicotine: The Constitution of the Alkaloids]. Berichte der Deutschen Chemischen Gesellschaft (in German). 26: 292–305. doi:10.1002/cber.18930260165.
- Pinner A (1893). "Ueber Nicotin. I. Mitteilung". Archiv der Pharmazie. 231 (5–6): 378–448. doi:10.1002/ardp.18932310508.
- Pictet A, Rotschy A (1904). "Synthese des Nicotins" [Synthesis of nicotine]. Berichte der Deutschen Chemischen Gesellschaft (in German). 37 (2): 1225–1235. doi:10.1002/cber.19040370206.
- Connolly GN, Alpert HR, Wayne GF, Koh H (October 2007). "Trends in nicotine yield in smoke and its relationship with design characteristics among popular US cigarette brands, 1997–2005". Tobacco Control. 16 (5): e5. doi:10.1136/tc.2006.019695. PMC 2598548. PMID 17897974.
- Mineur YS, Picciotto MR (December 2010). "Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis". Trends in Pharmacological Sciences. 31 (12): 580–6. doi:10.1016/j.tips.2010.09.004. PMC 2991594. PMID 20965579.
- Salín-Pascual RJ, Rosas M, Jimenez-Genchi A, Rivera-Meza BL, Delgado-Parra V (September 1996). "Antidepressant effect of transdermal nicotine patches in nonsmoking patients with major depression". The Journal of Clinical Psychiatry. 57 (9): 387–9. PMID 9746444.
- Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C (December 2008). "Smoking, dementia and cognitive decline in the elderly, a systematic review". BMC Geriatrics. 8: 36. doi:10.1186/1471-2318-8-36. PMC 2642819. PMID 19105840.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Henningfield JE, Zeller M (2009). "Nicotine Psychopharmacology: Policy and Regulatory". Nicotine Psychopharmacology. Handbook of Experimental Pharmacology. Vol. 192. pp. 511–34. doi:10.1007/978-3-540-69248-5_18. ISBN 978-3-540-69246-1. PMID 19184661.
- Grizzell JA, Echeverria V (October 2015). "New Insights into the Mechanisms of Action of Cotinine and its Distinctive Effects from Nicotine". Neurochemical Research. 40 (10): 2032–46. doi:10.1007/s11064-014-1359-2. PMID 24970109.
- Crooks PA, Dwoskin LP (October 1997). "Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking". Biochemical Pharmacology. 54 (7): 743–53. doi:10.1016/s0006-2952(97)00117-2. PMID 9353128.
- ^ Barreto GE, Iarkov A, Moran VE (January 2015). "Beneficial effects of nicotine, cotinine and its metabolites as potential agents for Parkinson's disease". Frontiers in Aging Neuroscience. 6: 340. doi:10.3389/fnagi.2014.00340. PMC 4288130. PMID 25620929.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Nielsen SS, Franklin GM, Longstreth WT, Swanson PD, Checkoway H (September 2013). "Nicotine from edible Solanaceae and risk of Parkinson disease". Annals of Neurology. 74 (3): 472–7. doi:10.1002/ana.23884. PMC 4864980. PMID 23661325.
Further reading
- Bilkei-Gorzo A, Rácz I, Michel K, Darvas M, Maldonado R, Zimmer A (January 2008). "A common genetic predisposition to stress sensitivity and stress-induced nicotine craving". Biological Psychiatry. 63 (2): 164–71. doi:10.1016/j.biopsych.2007.02.010. PMID 17570348.
- Gorrod, John W.; Peyton, Jacob, eds. (16 November 1999). Analytical Determination of Nicotine and Related Compounds and their Metabolites. Amsterdam: Elsevier. ISBN 978-0-08-052551-8.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Willoughby JO, Pope KJ, Eaton V (September 2003). "Nicotine as an antiepileptic agent in ADNFLE: an N-of-one study". Epilepsia. 44 (9): 1238–40. doi:10.1046/j.1528-1157.2003.11903.x. PMID 12919397.
- Minna JD (January 2003). "Nicotine exposure and bronchial epithelial cell nicotinic acetylcholine receptor expression in the pathogenesis of lung cancer". The Journal of Clinical Investigation. 111 (1): 31–3. doi:10.1172/JCI17492. PMC 151841. PMID 12511585.
- Fallon JH, Keator DB, Mbogori J, Taylor D, Potkin SG (March 2005). "Gender: a major determinant of brain response to nicotine". The International Journal of Neuropsychopharmacology. 8 (1): 17–26. doi:10.1017/S1461145704004730. PMID 15579215.
- West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA (January 2003). "Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells". The Journal of Clinical Investigation. 111 (1): 81–90. doi:10.1172/JCI16147. PMC 151834. PMID 12511591.
- National Institute on Drug Abuse
- Mayer B (January 2014). "How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century". Archives of Toxicology. 88 (1): 5–7. doi:10.1007/s00204-013-1127-0. PMC 3880486. PMID 24091634.
- Thomas GA, Rhodes J, Ingram JR (November 2005). "Mechanisms of disease: nicotine--a review of its actions in the context of gastrointestinal disease". Nature Clinical Practice. Gastroenterology & Hepatology. 2 (11): 536–44. doi:10.1038/ncpgasthep0316. PMID 16355159.
External links
- Description of nicotine mechanisms
- Erowid Nicotine Vault : Nicotine Material Safety Data Sheet
- CDC – NIOSH Pocket Guide to Chemical Hazards
| Reinforcement disorders: addiction and dependence | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Addiction |
| ||||||||||
| Dependence |
| ||||||||||
| Treatment and management |
| ||||||||||
| See also | |||||||||||
| Stimulants | |
|---|---|
| Adamantanes | |
| Adenosine antagonists | |
| Alkylamines | |
| Ampakines | |
| Arylcyclohexylamines | |
| Benzazepines | |
| Cathinones |
|
| Cholinergics |
|
| Convulsants | |
| Eugeroics | |
| Oxazolines | |
| Phenethylamines |
|
| Phenylmorpholines | |
| Piperazines | |
| Piperidines |
|
| Pyrrolidines | |
| Racetams | |
| Tropanes |
|
| Tryptamines | |
| Others |
|
| ATC code: N06B | |
| Drugs which induce euphoria | |
|---|---|
| |
| See also: Recreational drug use |
| Treatment of drug dependence (N07B) | |
|---|---|
| Nicotine dependence |
|
| Alcohol dependence | |
| Opioid dependence | |
| Benzodiazepine dependence |
|
| Cigarettes | |||
|---|---|---|---|
| Types | |||
| Components | |||
| Peripherals | |||
| Culture | |||
| Health issues | |||
| Related products | |||
| Tobacco industry |
| ||
| Government and the law | |||
| Lists | |||
| Pharmacodynamics | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||