| Antipsychotic | |
|---|---|
| Drug class | |
 Olanzapine, an example of a second-generation (atypical) antipsychotic Olanzapine, an example of a second-generation (atypical) antipsychotic | |
| Class identifiers | |
| Synonyms | Neuroleptics, major tranquilizers |
| Use | Principally: Schizophrenia, Schizoaffective disorder, Dementia, Tourette syndrome, Bipolar disorder, irritability in autism spectrum disorder |
| Clinical data | |
| Drugs.com | Drug Classes |
| External links | |
| MeSH | D014150 |
| Legal status | |
| In Wikidata | |
Antipsychotics, previously known as neuroleptics and major tranquilizers, are a class of psychotropic medication primarily used to manage psychosis (including delusions, hallucinations, paranoia or disordered thought), principally in schizophrenia but also in a range of other psychotic disorders. They are also the mainstay, together with mood stabilizers, in the treatment of bipolar disorder. Moreover, they are also used as adjuncts in the treatment of treatment-resistant major depressive disorder.
Use of any antipsychotic is associated with reductions in brain tissue volumes, including white matter reduction, an effect which is dose-dependent and time-dependent. A recent controlled trial suggests that second generation antipsychotics combined with intensive psychosocial therapy may potentially prevent pallidal brain volume loss in first episode psychosis.
The use of antipsychotics may result in many unwanted side effects such as involuntary movement disorders, gynecomastia, impotence, weight gain and metabolic syndrome. Long-term use can produce adverse effects such as tardive dyskinesia, tardive dystonia, and tardive akathisia.
First-generation antipsychotics (e.g., chlorpromazine), known as typical antipsychotics, were first introduced in the 1950s, and others were developed until the early 1970s. Second-generation antipsychotics, known as atypical antipsychotics, arrived with the introduction of clozapine in the early 1970s followed by others (e.g., risperidone). Both generations of medication block receptors in the brain for dopamine, but atypicals block serotonin receptors as well. Third-generation antipsychotics were introduced in the 2000s and offer partial agonism, rather than blockade, of dopamine receptors. Neuroleptic, originating from Ancient Greek: νεῦρον (neuron) and λαμβάνω (take hold of)—thus meaning "which takes the nerve"—refers to both common neurological effects and side effects.
Medical uses
Antipsychotics are most frequently used for the following conditions:
- Schizophrenia
- Schizoaffective disorder most commonly in conjunction with either an antidepressant (in the case of the depressive subtype) or a mood stabilizer (in the case of the bipolar subtype). Antipsychotics possess mood stabilizing properties and thus they may be used as standalone medication to treat mood dysregulation.
- Bipolar disorder (acute mania and mixed episodes) may be treated with either typical or atypical antipsychotics, although atypical antipsychotics are usually preferred because they tend to have more favourable adverse effect profiles and, according to a recent meta-analysis, they tend to have a lower liability for causing conversion from mania to depression.
- Psychotic depression. In this indication it is a common practice for the psychiatrist to prescribe a combination of an atypical antipsychotic and an antidepressant as this practice is best supported by the evidence.
- Treatment-resistant depression as an adjunct to standard antidepressant therapy.
Given the limited options available to treat the behavioral problems associated with dementia, other pharmacological and non-pharmacological interventions are usually attempted before using antipsychotics. A risk-to-benefit analysis is performed to weigh the risk of the adverse effects of antipsychotics versus: the potential benefit, the adverse effects of alternative interventions, and the risk of failing to intervene when a patient's behavior becomes unsafe. The same can be said for insomnia, in which they are not recommended as first-line therapy. There are evidence-based indications for using antipsychotics in children (e.g., tic disorder, bipolar disorder, psychosis), but the use of antipsychotics outside of those contexts (e.g., to treat behavioral problems) warrants significant caution.
Antipsychotics are used to treat tics associated with Tourette syndrome. Aripiprazole, an atypical antipsychotic, is used as add-on medication to ameliorate sexual dysfunction as a symptom of selective serotonin reuptake inhibitor (SSRI) antidepressants in women. Quetiapine is used to treat generalized anxiety disorder.
Schizophrenia
Main article: Schizophrenia § MedicationAntipsychotic drug treatment is a key component of schizophrenia treatment recommendations by the National Institute of Health and Care Excellence (NICE), the American Psychiatric Association, and the British Society for Psychopharmacology. The main aim of treatment with antipsychotics is to reduce the positive symptoms of psychosis, that include delusions and hallucinations. There is mixed evidence to support a significant impact of antipsychotic use on primary negative symptoms (such as apathy, lack of emotional affect, and lack of interest in social interactions) or on cognitive symptoms (memory impairments, reduced ability to plan and execute tasks). In general, the efficacy of antipsychotic treatment in reducing positive symptoms appears to increase with the severity of baseline symptoms. All antipsychotic medications work relatively the same way: by antagonizing D2 dopamine receptors. However, there are some differences when it comes to typical and atypical antipsychotics. For example, atypical antipsychotic medications have been seen to lower the neurocognitive impairment associated with schizophrenia more than conventional antipsychotics, although the reasoning and mechanics of this are still unclear to researchers.
Applications of antipsychotic drugs in the treatment of schizophrenia include prophylaxis for those showing symptoms that suggest that they are at high risk of developing psychosis; treatment of first-episode psychosis; maintenance therapy (a form of prophylaxis, maintenance therapy aims to maintain therapeutic benefit and prevent symptom relapse); and treatment of recurrent episodes of acute psychosis. A recent 2024 study found that using high doses of antipsychotics for schizophrenia was linked to a higher risk of mortality. Researchers analyzed data from 32,240 individuals aged 17 to 64 diagnosed with schizophrenia between 2002 and 2012 to arrive at this conclusion.
Prevention of psychosis and symptom improvement
Test batteries such as the PACE (Personal Assessment and Crisis Evaluation Clinic) and COPS (Criteria of Prodromal Syndromes), which measure low-level psychotic symptoms and cognitive disturbances, are used to evaluate people with early, low-level symptoms of psychosis. Test results are combined with family history information to identify patients in the "high-risk" group; they are considered to have a 20–40% risk of progression to frank psychosis within two years. These patients are often treated with low doses of antipsychotic drugs with the goal of reducing their symptoms and preventing progression to frank psychosis. While generally useful for reducing symptoms, clinical trials to date show little evidence that early use of antipsychotics improves long-term outcomes in those with prodromal symptoms, either alone or in combination with cognitive-behavioral therapy.
First-episode psychosis
First-episode psychosis (FEP) is the first time that psychotic symptoms are presented. NICE recommends that all people presenting with first-episode psychosis be treated with both an antipsychotic drug and cognitive behavioral therapy (CBT). NICE further recommends that those expressing a preference for CBT alone be informed that combination treatment is more effective. A diagnosis of schizophrenia is not made at this time as it takes longer to be determined by both DSM-5 and ICD-11, and only around 60% of those presenting with a first episode of psychosis will later be diagnosed with schizophrenia.
The conversion rate for a first episode of drug induced psychosis to bipolar disorder or schizophrenia is lower, with 30% of people converting to either bipolar disorder or schizophrenia. NICE makes no distinction between substance-induced psychosis and any other form of psychosis. The rate of conversion differs for different classes of drugs.
Pharmacological options for the specific treatment of FEP have been discussed in recent reviews. The goals of treatment for FEP include reducing symptoms and potentially improving long-term treatment outcomes. Randomized clinical trials have provided evidence for the efficacy of antipsychotic drugs in achieving the former goal, with first-generation and second generation antipsychotics showing about equal efficacy. The evidence that early treatment has a favorable effect on long-term outcomes is equivocal.
Recurrent psychotic episodes
Placebo-controlled trials of both first- and second-generation antipsychotic drugs consistently demonstrate the superiority of active drugs over placebos in suppressing psychotic symptoms. A large meta-analysis of 38 trials of antipsychotic drugs in schizophrenia with acute psychotic episodes showed an effect size of about 0.5. There is little or no difference in efficacy among approved antipsychotic drugs, including both first- and second-generation agents. The efficacy of such drugs is suboptimal. Few patients achieve complete resolution of symptoms. Response rates, calculated using various cutoff values for symptom reduction, are low, and their interpretation is complicated by high placebo response rates and selective publication of clinical trial results.
Maintenance therapy
The majority of patients treated with an antipsychotic drug will experience a response within four weeks. The goals of continuing treatment are to maintain suppression of symptoms, prevent relapse, improve quality of life, and support engagement in psychosocial therapy.
Maintenance therapy with antipsychotic drugs is clearly superior to placebo in preventing relapse but is associated with weight gain, movement disorders, and high dropout rates. A 3-year trial following persons receiving maintenance therapy after an acute psychotic episode found that 33% obtained long-lasting symptom reduction, 13% achieved remission, and only 27% experienced satisfactory quality of life. The effect of relapse prevention on long term outcomes is uncertain, as historical studies show little difference in long term outcomes before and after the introduction of antipsychotic drugs.
While maintenance therapy clearly reduces the rate of relapses requiring hospitalization, a large observational study in Finland found that, in people that eventually discontinued antipsychotics, the risk of being hospitalized again for a mental health problem or dying increased the longer they were dispensed (and presumably took) antipsychotics prior to stopping therapy. If people did not stop taking antipsychotics, they remained at low risk for relapse and hospitalization compared to those that did. The authors speculated that the difference may be because the people that discontinued treatment after a longer time had more severe mental illness than those that discontinued antipsychotic therapy sooner.
A significant challenge in the use of antipsychotic drugs for the prevention of relapse is the poor rate of adherence. In spite of the relatively high rates of adverse effects associated with these drugs, some evidence, including higher dropout rates in placebo arms compared to treatment arms in randomized clinical trials, suggests that most patients who discontinue treatment do so because of suboptimal efficacy. If someone experiences psychotic symptoms due to nonadherence, they may be compelled to receive treatment through a process called involuntary commitment, in which they can be forced to accept treatment (including antipsychotics). A person can also be committed to treatment outside of a hospital, called outpatient commitment.
Antipsychotics in long-acting injectable (LAI), or "depot", form have been suggested as a method of decreasing medication nonadherence (sometimes also called non-compliance). NICE advises LAIs be offered to patients when preventing covert, intentional nonadherence is a clinical priority. LAIs are used to ensure adherence in outpatient commitment. A meta-analysis found that LAIs resulted in lower rates of rehospitalization with a hazard ratio of 0.83; however, these results were not statistically significant (the 95% confidence interval was 0.62 to 1.11).
Bipolar disorder
Main article: Bipolar disorderAntipsychotics are routinely used, often in conjunction with mood stabilizers such as lithium/valproate, as a first-line treatment for manic and mixed episodes associated with bipolar disorder. The reason for this combination is the therapeutic delay of the aforementioned mood stabilizers (for valproate therapeutic effects are usually seen around five days after treatment is commenced whereas lithium usually takes at least a week before the full therapeutic effects are seen) and the comparatively rapid antimanic effects of antipsychotic drugs. The antipsychotics have a documented efficacy when used alone in acute mania/mixed episodes.
At least five atypical antipsychotics (lumateperone, cariprazine, lurasidone, olanzapine, and quetiapine) have also been found to possess efficacy in the treatment of bipolar depression as a monotherapy, whereas only olanzapine and quetiapine have been proven to be effective broad-spectrum (i.e., against all three types of relapse—manic, mixed and depressive) prophylactic (or maintenance) treatments in patients with bipolar disorder. A recent Cochrane review also found that olanzapine had a less favourable risk/benefit ratio than lithium as a maintenance treatment for bipolar disorder.
The American Psychiatric Association and the UK National Institute for Health and Care Excellence recommend antipsychotics for managing acute psychotic episodes in schizophrenia or bipolar disorder, and as a longer-term maintenance treatment for reducing the likelihood of further episodes. They state that response to any given antipsychotic can be variable so that trials may be necessary, and that lower doses are to be preferred where possible. A number of studies have looked at levels of "compliance" or "adherence" with antipsychotic regimes and found that discontinuation (stopping taking them) by patients is associated with higher rates of relapse, including hospitalization.
Dementia
Psychosis and agitation develop in as many as 80 percent of people living in nursing homes. Despite a lack of FDA approval and black-box warnings, atypical antipsychotics are very often prescribed to people with dementia. An assessment for an underlying cause of behavior is needed before prescribing antipsychotic medication for symptoms of dementia. Antipsychotics in old age dementia showed a modest benefit compared to placebo in managing aggression or psychosis, but this is combined with a fairly large increase in serious adverse events. Thus, antipsychotics should not be used routinely to treat dementia with aggression or psychosis, but may be an option in a few cases where there is severe distress or risk of physical harm to others. Psychosocial interventions may reduce the need for antipsychotics. In 2005, the FDA issued an advisory warning of an increased risk of death when atypical antipsychotics are used in dementia. In the subsequent 5 years, the use of atypical antipsychotics to treat dementia decreased by nearly 50%.
Major depressive disorder
A number of atypical antipsychotics have some benefits when used in addition to other treatments in major depressive disorder. Aripiprazole, quetiapine extended-release, and olanzapine (when used in conjunction with fluoxetine) have received the Food and Drug Administration (FDA) labelling for this indication. There is, however, a greater risk of side effects with their use compared to using traditional antidepressants. The greater risk of serious side effects with antipsychotics is why, e.g., quetiapine was denied approval as monotherapy for major depressive disorder or generalized anxiety disorder, and instead was only approved as an adjunctive treatment in combination with traditional antidepressants.
A recent study on the use of antipychotics in unipolar depression concluded that the use of those drugs in addition to antidepressants alone leads to a worse disease outcome. This effect is especially pronounced in younger patients with psychotic unipolar depression. Considering the wide use of such combination therapies, further studies on the side effects of antipychotics as an add-on therapy are warranted.
Other
Global antipsychotic utilization has seen a steady growth since the introduction of atypical (second-generation) antipsychotics and this is ascribed to off-label use for many other unapproved disorders. Besides the above uses antipsychotics may be used for obsessive–compulsive disorder, post-traumatic stress disorder, personality disorders, Tourette syndrome, autism and agitation in those with dementia. Evidence however does not support the use of atypical antipsychotics in eating disorders or personality disorder. The atypical antipsychotic risperidone may be useful for obsessive–compulsive disorder. The use of low doses of antipsychotics for insomnia, while common, is not recommended as there is little evidence of benefit as well as concern regarding adverse effects. Some of the more serious adverse effects may also occur at the low doses used, such as dyslipidemia and neutropenia, and a recent network meta-analysis of 154 double-blind, randomized controlled trials of drug therapies vs. placebo for insomnia in adults found that quetiapine did not demonstrated any short-term benefits in sleep quality. Low dose antipsychotics may also be used in treatment of impulse-behavioural and cognitive-perceptual symptoms of borderline personality disorder. Despite the lack of evidence supporting the benefit of antipsychotics in people with personality disorders, 1 in 4 who do not have a serious mental illness are prescribed them in UK primary care. Many people receive these medication for over a year, contrary to NICE guidelines.
In children they may be used in those with disruptive behavior disorders, mood disorders and pervasive developmental disorders or intellectual disability. Antipsychotics are only weakly recommended for Tourette syndrome, because although they are effective, side effects are common. The situation is similar for those on the autism spectrum. Much of the evidence for the off-label use of antipsychotics (for example, for dementia, OCD, PTSD, personality disorders, Tourette's) was of insufficient scientific quality to support such use, especially as there was strong evidence of increased risks of stroke, tremors, significant weight gain, sedation, and gastrointestinal problems. A UK review of unlicensed usage in children and adolescents reported a similar mixture of findings and concerns. A survey of children with pervasive developmental disorder found that 16.5% were taking an antipsychotic drug, most commonly for irritability, aggression, and agitation. Both risperidone and aripiprazole have been approved by the US FDA for the treatment of irritability in autistic children and adolescents. A review in the UK found that the use of antipsychotics in England doubled between 2000 and 2019. Children were prescribed antipsychotics for conditions for which there is no approval, such as autism.
Aggressive challenging behavior in adults with intellectual disability is often treated with antipsychotic drugs despite lack of an evidence base. A recent randomized controlled trial, however, found no benefit over placebo and recommended that the use of antipsychotics in this way should no longer be regarded as an acceptable routine treatment.
Antipsychotics may be an option, together with stimulants, in people with ADHD and aggressive behavior when other treatments have not worked. They have not been found to be useful for the prevention of delirium among those admitted to hospital.
Typicals vs atypicals
Aside from reduced extrapyramidal symptoms, and with the clear exception of clozapine, it is unclear whether the atypical (second-generation) antipsychotics offer advantages over older, first generation antipsychotics. Amisulpride, olanzapine, risperidone and clozapine may be more effective but are associated with greater side effects. Typical antipsychotics have equal drop-out and symptom relapse rates to atypicals when used at low to moderate dosages.
Clozapine is an effective treatment for those who respond poorly to other drugs ("treatment-resistant" or "refractory" schizophrenia), but it has the potentially serious side effect of agranulocytosis (lowered white blood cell count) in less than 4% of people.
Due to bias in the research the accuracy of comparisons of atypical antipsychotics is a concern.
In 2005, a US government body, the National Institute of Mental Health published the results of a major independent study (the CATIE project). No other atypical studied (risperidone, quetiapine, and ziprasidone) did better than the first-generation antipsychotic perphenazine on the measures used, nor did they produce fewer adverse effects than the typical antipsychotic perphenazine, although more patients discontinued perphenazine owing to extrapyramidal effects compared to the atypical agents (8% vs. 2% to 4%). This is significant because any patient with tardive dyskinesia was specifically excluded from randomization to perphenazine; i.e., in the CATIE study the patient cohort randomized to receive perphenazne was at lower risk of having extrapyramidal symptoms.
Atypical antipsychotics do not appear to lead to improved rates of medication adherence compared to typical antipsychotics.
Many researchers question the first-line prescribing of atypicals over typicals, and some even question the distinction between the two classes. In contrast, other researchers point to the significantly higher risk of tardive dyskinesia and other extrapyramidal symptoms with the typicals and for this reason alone recommend first-line treatment with the atypicals, notwithstanding a greater propensity for metabolic adverse effects in the latter. The UK government organization NICE recently revised its recommendation favoring atypicals, to advise that the choice should be an individual one based on the particular profiles of the individual drug and on the patient's preferences.
The re-evaluation of the evidence has not necessarily slowed the bias toward prescribing the atypicals.
Other uses
Antipsychotics, such as risperidone, quetiapine, and olanzapine, have been used as hallucinogen antidotes or "trip killers" to block the effects of serotonergic psychedelics like psilocybin and lysergic acid diethylamide (LSD).
Adverse effects
For more detailed comparison of atypical antipsychotics, see Atypical antipsychotic § Adverse effects.Generally, more than one antipsychotic drug should not be used at a time because of increased adverse effects.
Some atypicals are associated with considerable weight gain, diabetes and the risk of metabolic syndrome. Unwanted side effects cause people to stop treatment, resulting in relapses. Risperidone (atypical) has a similar rate of extrapyramidal symptoms to haloperidol (typical). A rare but potentially lethal condition of neuroleptic malignant syndrome (NMS) has been associated with the use of antipsychotics. Through its early recognition, and timely intervention rates have declined. However, an awareness of the syndrome is advised to enable intervention. Another less rare condition of tardive dyskinesia can occur due to long-term use of antipsychotics, developing after months or years of use. It is more often reported with use of typical antipsychotics. Very rarely antipsychotics may cause tardive psychosis.
Clozapine is associated with side effects that include weight gain, tiredness, and hypersalivation. More serious adverse effects include seizures, NMS, neutropenia, and agranulocytosis (lowered white blood cell count) and its use needs careful monitoring.
Clozapine is also associated with thromboembolism (including pulmonary embolism), myocarditis, and cardiomyopathy. A systematic review of clozapine-associated pulmonary embolism indicates that this adverse effect can often be fatal, and that it has an early onset, and is dose-dependent. The findings advised the consideration of using a prevention therapy for venous thromboembolism after starting treatment with clozapine, and continuing this for six months. Constipation is three times more likely to occur with the use of clozapine, and severe cases can lead to ileus and bowel ischemia resulting in many fatalities. Very rare clozapine adverse effects include periorbital edema due to several possible mechanisms (e.g., inhibition of platelet-derived growth factor receptors leading to increased vascular permeability, antagonism of renal dopamine receptors with electrolyte and fluid imbalance and immune-mediated hypersensitivity reactions).
However, the risk of serious adverse effects from clozapine is low, and there are the beneficial effects to be gained of a reduced risk of suicide, and aggression. Typical antipsychotics and atypical risperidone can have a side effect of sexual dysfunction. Clozapine, olanzapine, and quetiapine are associated with beneficial effects on sexual functioning helped by various psychotherapies.
By rate
Common (≥ 1% and up to 50% incidence for most antipsychotic drugs) adverse effects of antipsychotics include:
- Dysphoria and apathy (due to dopamine receptor blockade)
- Sedation (particularly common with asenapine, clozapine, olanzapine, quetiapine, chlorpromazine and zotepine)
- Headaches
- Dizziness
- Diarrhea
- Anxiety
- Extrapyramidal side effects (particularly common with first-generation antipsychotics), which include:
- Akathisia, an often distressing sense of inner restlessness.
- Dystonia, an abnormal muscle contraction
- Pseudoparkinsonism, symptoms that are similar to what people with Parkinson's disease experience, including tremulousness and drooling
- Hyperprolactinaemia (rare for those treated with clozapine, quetiapine and aripiprazole), which can cause:
- Galactorrhoea, the unusual secretion of breast milk.
- Gynaecomastia, abnormal growth of breast tissue
- Sexual dysfunction (in both sexes)
- Osteoporosis
- Orthostatic hypotension
- Weight gain (particularly prominent with clozapine, olanzapine, quetiapine and zotepine, can be counteracted by starting the drug with metformin)
- Anticholinergic side-effects (common for olanzapine, clozapine; less likely on risperidone) such as:
- Blurred vision
- Constipation
- Dry mouth (although hypersalivation may also occur)
- Reduced perspiration
- Tardive dyskinesia appears to be more frequent with high-potency first-generation antipsychotics, such as haloperidol, and tends to appear after chronic and not acute treatment. It is characterized by slow (hence the tardive) repetitive, involuntary and purposeless movements, most often of the face, lips, legs, or torso, which tend to resist treatment and are frequently irreversible. The rate of appearance of TD is about 5% per year of use of antipsychotic drug (whatever the drug used)
- Breast cancer: a systematic review and meta-analysis of observational studies with over 2 million individuals estimated an association between antipsychotic use and breast cancer by over 30%.
Rare/Uncommon (<1% incidence for most antipsychotic drugs) adverse effects of antipsychotics include:
- Blood dyscrasias (e.g., agranulocytosis, leukopenia, and neutropaenia), which is more common in patients on clozapine.
- Metabolic syndrome and other metabolic problems such as type II diabetes mellitus — particularly common with clozapine, olanzapine and zotepine. In American studies African Americans appeared to be at a heightened risk for developing type II diabetes mellitus. Evidence suggests that females are more sensitive to the metabolic side effects of first-generation antipsychotic drugs than males. Metabolic adverse effects appear to be mediated by antagonizing the histamine H1 and serotonin 5-HT2C receptors and perhaps by interacting with other neurochemical pathways in the central nervous system.
- Neuroleptic malignant syndrome, a potentially fatal condition characterized by:
- Autonomic instability, which can manifest with tachycardia, nausea, vomiting, diaphoresis, etc.
- Hyperthermia — elevated body temperature.
- Mental status change (confusion, hallucinations, coma, etc.)
- Muscle rigidity
- Laboratory abnormalities (e.g., elevated creatine kinase, reduced iron plasma levels, electrolyte abnormalities, etc.)
- Pancreatitis
- QT interval prolongation — more prominent in those treated with amisulpride, pimozide, sertindole, thioridazine and ziprasidone.
- Torsades de pointes
- Seizures, particularly in people treated with chlorpromazine and clozapine.
- Thromboembolism
- Myocardial infarction
- Stroke
- Pisa syndrome
Long-term effects
See also: List of long term side effects of antipsychoticsSome studies have found decreased life expectancy associated with the use of antipsychotics, and argued that more studies are needed. Antipsychotics may also increase the risk of early death in individuals with dementia. Antipsychotics typically worsen symptoms in people with depersonalisation disorder. Antipsychotic polypharmacy (prescribing two or more antipsychotics at the same time for an individual) is a common practice but not evidence-based or recommended, and there are initiatives to curtail it. Similarly, the use of excessively high doses (often the result of polypharmacy) continues despite clinical guidelines and evidence indicating that it is usually no more effective but is usually more harmful. A meta-analysis of observational studies with over two million individuals has suggested a moderate association of antipsychotic use with breast cancer.
Loss of grey matter and other brain structural changes over time are observed amongst people diagnosed with schizophrenia. Meta-analyses of the effects of antipsychotic treatment on grey matter volume and the brain's structure have reached conflicting conclusions. A 2012 meta-analysis concluded that grey matter loss is greater in patients treated with first generation antipsychotics relative to those treated with atypicals, and hypothesized a protective effect of atypicals as one possible explanation. A second meta-analysis suggested that treatment with antipsychotics was associated with increased grey matter loss. Animal studies found that monkeys exposed to both first- and second-generation antipsychotics experience significant reduction in brain volume, resulting in an 8-11% reduction in brain volume over a 17–27 month period.
The National Association of State Mental Health Program Directors said that antipsychotics are not interchangeable and it is recommend including trying at least one weight-neutral treatment for those patients with potential metabolic issues.
Subtle, long-lasting forms of akathisia are often overlooked or confused with post-psychotic depression, in particular when they lack the extrapyramidal aspect that psychiatrists have been taught to expect when looking for signs of akathisia.
Adverse effect on cognitive function and increased risk of death in people with dementia along with worsening of symptoms has been described in the literature.
Antipsychotics, due to acting as dopamine D2 receptor antagonists and thereby stimulating pituitary lactotrophs, may have a risk of prolactinoma with long-term use. This is also responsible for their induction of hyperprolactinemia (high prolactin levels).
Discontinuation
See also: Drug withdrawal § Prescription medicineThe British National Formulary recommends a gradual withdrawal when discontinuing antipsychotics to avoid acute withdrawal syndrome or rapid relapse. Symptoms of withdrawal commonly include nausea, vomiting, and loss of appetite. Other symptoms may include restlessness, increased sweating, and trouble sleeping. Less commonly there may be a feeling of the world spinning, numbness, or muscle pains. Symptoms generally resolve after a short period of time.
There is tentative evidence that discontinuation of antipsychotics can result in psychosis. It may also result in recurrence of the condition that is being treated. Rarely, tardive dyskinesia can occur when the medication is stopped.
Unexpected psychotic episodes have been observed in patients withdrawing from clozapine. This is referred to as supersensitivity psychosis, not to be equated with tardive dyskinesia.
Tardive dyskinesia may abate during withdrawal from the antipsychotic agent, or it may persist.
Withdrawal effects may also occur when switching a person from one antipsychotic to another, (it is presumed due to variations of potency and receptor activity). Such withdrawal effects can include cholinergic rebound, an activation syndrome, and motor syndromes including dyskinesias. These adverse effects are more likely during rapid changes between antipsychotic agents, so making a gradual change between antipsychotics minimises these withdrawal effects. The British National Formulary recommends a gradual dose reduction when discontinuing antipsychotic treatment to avoid acute withdrawal symptoms or rapid relapse. The process of cross-titration involves gradually increasing the dose of the new medication while gradually decreasing the dose of the old medication.
City and Hackney Clinical Commissioning Group found more than 1,000 patients in their area in July 2019 who had not had regular medication reviews or health checks because they were not registered as having serious mental illness. On average they had been taking these drugs for six years. If this is typical of practice in England more than 100,000 patients are probably in the same position.
List of agents
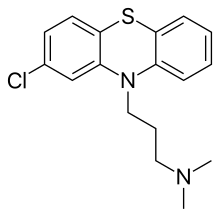

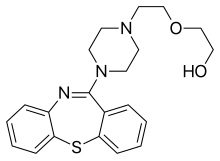
Clinically used antipsychotic medications are listed below by drug group. Trade names appear in parentheses. A 2013 review has stated that the division of antipsychotics into first and second generation is perhaps not accurate.
Notes:
† indicates drugs that are no longer (or were never) marketed in English-speaking countries.
‡ denotes drugs that are no longer (or were never to begin with) marketed in the United States. Some antipsychotics are not firmly placed in either first-generation or second-generation classes.
# denotes drugs that have been withdrawn worldwide.
First-generation (typical)
Main article: Typical antipsychoticButyrophenones
Main article: Butyrophenones- Benperidol
- Bromperidol
- Droperidol
- Haloperidol (Haldol)
- Moperone (discontinued)
- Pipamperone (discontinued)
- Timiperone
Diphenylbutylpiperidines
Phenothiazines
Main article: Phenothiazines- Acepromazine — although it is mostly used in veterinary medicine.
- Chlorpromazine (Thorazine)
- Cyamemazine
- Dixyrazine
- Fluphenazine
- Levomepromazine
- Mesoridazine (discontinued)
- Perazine
- Pericyazine
- Perphenazine
- Pipotiazine
- Prochlorperazine
- Promazine (discontinued)
- Promethazine
- Prothipendyl
- Thioproperazine (only English-speaking country it is available in is Canada)
- Thioridazine (discontinued)
- Trifluoperazine
- Triflupromazine (discontinued)
Thioxanthenes
Main article: ThioxanthenesDisputed/unknown
This category is for drugs that have been called both first and second-generation, depending on the literature being used.
Benzamides
Tricyclics
Others
Second-generation (atypical)
Main article: Atypical antipsychoticBenzamides
- Amisulpride (Socian) – Selective dopamine antagonist. Higher doses (greater than 400 mg) act upon post-synaptic dopamine receptors resulting in a reduction in the positive symptoms of schizophrenia, such as psychosis. Lower doses, however, act upon dopamine autoreceptors, resulting in increased dopamine transmission, improving the negative symptoms of schizophrenia. Lower doses of amisulpride have also been shown to have antidepressant and anxiolytic effects in non-schizophrenic patients, leading to its use in dysthymia and social phobias.
- Nemonapride – Used in Japan.
- Remoxipride – Has a risk of causing aplastic anaemia and, hence, has been withdrawn from the market worldwide. It has also been found to possess relatively low (virtually absent) potential to induce hyperprolactinaemia and extrapyramidal symptoms, likely attributable to its comparatively weak binding to (and, hence, rapid dissociation from) the D2 receptor.
- Sultopride – An atypical antipsychotic of the benzamide chemical class used in Europe, Japan, and Hong Kong for the treatment of schizophrenia. It was launched by Sanofi-Aventis in 1976. Sultopride acts as a selective D2 and D3 receptor antagonist.
Benzisoxazoles/benzisothiazoles
- Iloperidone (Fanapt) – Approved by the US FDA in 2009, it is fairly well tolerated, although hypotension, dizziness, and somnolence were very common side effects. Has not received regulatory approval in other countries, however.
- Paliperidone (Invega) – Primary, active metabolite of risperidone that was approved in 2006.
- Perospirone – Has a higher incidence of extrapyramidal side effects than other atypical antipsychotics.
- Risperidone (Risperdal) – Divided dosing is recommended until initial titration is completed, at which time the drug can be administered once daily. Used off-label to treat Tourette syndrome and anxiety disorder.
- Ziprasidone (Geodon) – Approved in 2004 to treat bipolar disorder. Side-effects include a prolonged QT interval in the heart, which can be dangerous for patients with heart disease or those taking other drugs that prolong the QT interval.
- Lurasidone (Latuda) – Approved by the US FDA for schizophrenia and bipolar depression, and for use as schizophrenia treatment in Canada.
Butyrophenones
- Melperone – Only used in a few European countries. No English-speaking country has licensed it to date.
- Lumateperone (Caplyta)
Tricyclics
- Asenapine (Saphris) – Of the dibenzo-oxepino pyrrole class of atypical antipsychotics. Used for the treatment of schizophrenia and acute mania associated with bipolar disorder.
- Clozapine (Clozaril) – Of the dibenzodiazepine class of atypical antipsychotics. Requires routine laboratory monitoring of complete blood counts every one to four weeks due to the risk of agranulocytosis. It has unparalleled efficacy in the treatment of treatment-resistant schizophrenia.
- Olanzapine (Zyprexa) – Of the theienobenzodiazepine class of atypical antipsychotics. Used to treat psychotic disorders including schizophrenia, acute manic episodes, and maintenance of bipolar disorder. Used as an adjunct to antidepressant therapy, either alone or in combination with fluoxetine as Symbyax.
- Quetiapine (Seroquel) – Of the dibenzothiazepine class of atypical antipsychotics. Used primarily to treat bipolar disorder and schizophrenia. Also used and licensed in a few countries (including Australia, the United Kingdom and the United States) as an adjunct to antidepressant therapy in patients with major depressive disorder. It's the only antipsychotic that's demonstrated efficacy as a monotherapy for the treatment of major depressive disorder and bipolar disorder (it treats mixed mood swings alone). It indirectly serves as a norepinephrine reuptake inhibitor by means of its active metabolite, norquetiapine.
- Zotepine – Of the dibenzothiepin class of atypical antipsychotic indicated for acute and chronic schizophrenia. It is still used in Japan and was once used in Germany but it was discontinued.
Others
- Blonanserin – Approved by the PMDA in 2008. Used in Japan and South Korea.
- Pimavanserin – A selective 5-HT2A receptor antagonist approved for the treatment of Parkinson's disease psychosis in 2016.
- Sertindole – Developed by the Danish pharmaceutical company H. Lundbeck. Like the other atypical antipsychotics, it is believed to have antagonist activity at dopamine and serotonin receptors in the brain.
Third-generation
Third generation antipsychotics are recognized as demonstrating D2 receptor partial agonism as opposed to the D2 and 5HT-2A receptor antagonism of second-generation (atypical) antipsychotics and D2 antagonism of first-generation (typical) antipsychotics.
Benzisoxazoles/benzisothiazoles
- Lumateperone (Caplyta) – In December 2019, lumateperone, a presynaptic D2 receptor partial agonist and postsynaptic D2 receptor antagonist, received its first global approval in the US for the treatment of schizophrenia in adults. In 2020 and 2021 FDA approved for depressive episodes associated with bipolar I or II disorder in adults, as monotherapy and as adjunctive therapy with lithium or valproate.
Phenylpiperazines/quinolinones/benzoxazinones
- Aripiprazole (Abilify) - Partial agonist at the D2 receptor. Considered the prototypical third-generation antipsychotic.
- Aripiprazole lauroxil (Abilify Maintena) – Long-acting version of aripiprazole for injection.
- Brexpiprazole (Rexulti) – Partial agonist of the D2 receptor. Successor of aripiprazole.
- Brilaroxazine – A D2/3/4 and 5-HT1A partial agonist and 5-HT2A/2B/7 antagonist
- Cariprazine (Vraylar, Reagila) – A D3-preferring D2/3 partial agonist.
Muscarinic agonists
- Xanomeline/trospium chloride (Cobenfy)
Mechanism of action
Antipsychotic drugs such as haloperidol and chlorpromazine tend to block dopamine D2 receptors in the dopaminergic pathways of the brain. This means that dopamine released in these pathways has less effect. Excess release of dopamine in the mesolimbic pathway has been linked to psychotic experiences. Decreased dopamine release in the prefrontal cortex, and excess dopamine release in other pathways, are associated with psychotic episodes in schizophrenia and bipolar disorder.
In addition to the antagonistic effects of dopamine, antipsychotics (in particular atypical neuroleptics) also antagonize 5-HT2A receptors. Different alleles of the 5-HT2A receptor have been associated with schizophrenia and other psychoses, including depression. Higher concentrations of 5-HT2A receptors in cortical and subcortical areas, in particular in the right caudate nucleus have been historically recorded.
Typical antipsychotics are not particularly selective and also block dopamine receptors in the mesocortical pathway, tuberoinfundibular pathway, and the nigrostriatal pathway. Blocking D2 receptors in these other pathways is thought to produce some unwanted side effects that the typical antipsychotics can produce (see above). They were commonly classified on a spectrum of low potency to high potency, where potency referred to the ability of the drug to bind to dopamine receptors, and not to the effectiveness of the drug. High-potency antipsychotics such as haloperidol, in general, have doses of a few milligrams and cause less sleepiness and calming effects than low-potency antipsychotics such as chlorpromazine and thioridazine, which have dosages of several hundred milligrams. The latter have a greater degree of anticholinergic and antihistaminergic activity, which can counteract dopamine-related side-effects.
Atypical antipsychotic drugs have a similar blocking effect on D2 receptors; however, most also act on serotonin receptors, especially 5-HT2A and 5-HT2C receptors. Both clozapine and quetiapine appear to bind just long enough to elicit antipsychotic effects but not long enough to induce extrapyramidal side effects and prolactin hypersecretion. 5-HT2A antagonism increases dopaminergic activity in the nigrostriatal pathway, leading to a lowered extrapyramidal side effect liability among the atypical antipsychotics.
Through the ability of most antipsychotics to antagonize 5-HT2A serotonin pathways enabling a sensitisation of postsynaptic serotonin receptors, MDMA exposure can be more intense because it has more excitatory receptors to activate. The same effect can be observed with the D2 antagonizing with normal amphetamine (with this just being hypothetical as there is the fact that antipsychotics sensitize receptors, with exact these postsynaptic receptors (5-HT2A, D2) being flooded by the respective neurotransmitter (serotonin, dopamine) from amphetamine exposure).
Comparison of medications
| Overview | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tolerability (as propensity for adverse effects) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Generic name |
Discontinuation rate
(OR with 95% CI) |
Anticholinergic effects | Sedation | EPSE | Weight Gain | Metabolic AEs | QTc prolongation
(ORs & 95% CIs) |
PE | Hypotension | Notes (e.g., notable AEs*) | ||
| Amisulpride | 0.43 (0.32–0.57) | - | - | + | + | +/- | +++ 0.66 (0.39–0.91) |
+++/++ | - | Torsades de Pointes common on overdose. Has a comparatively low penetrability of the blood–brain barrier. | ||
| Amoxapine | ? | ++ | ++ | +/- | ++/+ | ++/+ | ++/+ | ++/+ | ++/+ | Amoxapine is also an antidepressant. Very toxic in overdose due to the potential for renal failure and seizures. | ||
| Aripiprazole | 0.61 (0.51–0.72) | - | + | +/- (akathisia mostly) |
+ | +/- | - 0.01 (−0.13 to 0.15) |
- (can reduce prolactin levels) |
- | Only clinically utilised antipsychotic that does not act by antagonising the D2 receptor and rather partially agonises this receptor. | ||
| Asenapine | 0.69 (0.54–0.86) | - | ++ | + | + | +/- | ++/+ 0.30 (−0.04 to 0.65) |
+ | + | Oral hypoesthesia. Has a complex pharmacologic profile. | ||
| Blonanserin | ~0.7 | + | + | ++/+ | +/- | +/- | - | ++/+ | +/- | Only used in a few East Asian countries. | ||
| Chlorpromazine | 0.65 (0.5–0.84) | +++ | +++ | ++ | ++ | ++ | ++ | +++ | +++ | First marketed antipsychotic, sort of the prototypical low-potency first-generation (typical) antipsychotic. | ||
| Clozapine | 0.46 (0.32–0.65) | +++ | +++ | - | +++ | +++ | + | - | +++ | Notable AEs: Agranulocytosis, neutropaenia, leukopaenia and myocarditis. Dose-dependent seizure risk. Overall the most effective antipsychotic, on average. Usually reserved for treatment-resistant cases or highly suicidal patients. | ||
| Droperidol | ? | +/- | +/- | +++ | +/- | +/- | ? | +++ | ? | Mostly used for postoperative nausea and vomiting. | ||
| Flupenthixol | ? | ++ | + | ++ | ++ | ++ | + | +++ | + | Also used in lower doses for depression. | ||
| Fluphenazine | 0.69 (0.24–1.97) | ++ | + | +++ | + | + | + | +++ | + | High-potency first-generation (typical) antipsychotic. | ||
| Haloperidol | 0.8 (0.71–0.90) | + | + | +++ | + | +/- | + 0.11 (0.03–0.19) |
+++ | + | Prototypical high-potency first-generation (typical) antipsychotic. | ||
| Iloperidone | 0.69 (0.56–0.84) | - | +/- | + | ++ | ++ | ++ 0.34 (0.22–0.46) |
++/+ | + | ? | ||
| Levomepromazine | ? | +++ | +++ | ++/+ | ++ | ++ | ? | +++ | +++ | Also used as an analgesic, agitation, anxiety and emesis. | ||
| Loxapine | 0.52 (0.28–0.98) | + | ++ | +++ | + | +/- | ? | +++ | ++ | ? | ||
| Lurasidone | 0.77 (0.61–0.96) | - | - | ++/+ | - | - | - −0.10 (−0.21 to 0.01) |
++/+ | - | May be particularly helpful in ameloriating the cognitive symptoms of schizophrenia, likely due to its 5-HT7 receptor. | ||
| Melperone | ? | - | +/- | - | +/- | +/- | ++ | - | ++/+ | Several smaller low-quality clinical studies have reported its efficacy in the treatment of treatment-resistant schizophrenia. Only approved for use in a few European countries. It is known that off-licence prescribing of melperone is occurring in the United Kingdom. Is a butyrophenone, low-potency atypical antipsychotic that has been tried as a treatment for Parkinson's disease psychosis, although with negative results. | ||
| Molindone | ? | - | ++/+ | + | - | - | ? | +++ | +/- | Withdrawn from the market. Seems to promote weight loss (which is rather unusual for an antipsychotic seeing how they tend to promote weight gain). | ||
| Olanzapine | 0.46 (0.41–0.52) | + | ++ | + | +++ | +++ | + 0.22 (0.11–0.31) |
+ | + | ? | ||
| Paliperidone | 0.48 (0.39–0.58) | - | - | ++/+ (dose dependent) |
++ | + | – 0.05 (−0.18 to 0.26) |
+++ | ++ | Active metabolite of risperidone. | ||
| Perazine | 0.62 (0.4–1.10) | ? | ? | ? | ? | ? | ? | ? | ? | Limited data available on adverse effects. | ||
| Periciazine | ? | +++ | +++ | + | ++ | + | ? | +++ | ++ | Also used to treat severe anxiety. Not licensed for use in the US. | ||
| Perospirone | ? | +/- | + | ++/+ | +/- | ? | - | ++/+ | - | Usually grouped with the atypical antipsychotics despite its relatively high propensity for causing extrapyramidal side effects. | ||
| Perphenazine | 0.30 (0.04–2.33) | + | + | +++ | + | + | + | +++ | + | Has additional antiemetic effects. | ||
| Pimozide | 1.01 (0.30–3.39) | + | + | + | + | + | +++ | +++ | + | High potency first-generation (typical) antipsychotic. | ||
| Pipotiazine | ? | ++ | ++ | ++ | ++ | + | ? | +++ | ++ | Only available in the UK. | ||
| Prochlorperazine | ? | ? | ? | +++ | ? | ? | + | +++ | ? | Primarily used in medicine as an antiemetic. | ||
| Quetiapine | 0.61 (0.52–0.71) | ++/+ | ++ | - | ++ | ++/+ | + 0.17 (0.06–0.29) |
- | ++ | Binds to the D2 receptor in a hit and run fashion. That is it rapidly dissociates from said receptor and hence produces antipsychotic effects but does not bind to the receptor long enough to produce extrapyramidal side effects and hyperprolactinaemia. | ||
| Remoxipride | ? | - | +/- | - | +/- | +/- | - | - | - | Removed from the market amidst concerns about an alarmingly high rate of aplastic anaemia. | ||
| Risperidone | 0.53 (0.46–0.60) | - | ++/+ (dose-dependent) |
++ | ++ | ++/+ | ++ 0.25 (0.15–0.36) |
+++ | ++ | ? | ||
| Sertindole | 0.78 (0.61–0.98) | - | - | - | ++ | ++/+ | +++ 0.90 (0.76–1.02) |
- | +++ | Not licensed for use in the US. | ||
| Sulpiride | 1.00 (0.25–4.00) | - | - | + | + | +/- | + | +++/++ | - | Not licensed for use in the US. | ||
| Thioridazine | 0.67 (0.32–1.40) | +++ | +++ | + | ++ | ++ | +++ | +++ | +++ | Dose-dependent risk for degenerative retinopathies. Found utility in reducing the resistance of multidrug and even extensively resistant strains of tuberculosis to antibiotics. | ||
| Tiotixene | ? | - | + | +++ | ++ | ++/+ | + | +++ | + | ? | ||
| Trifluoperazine | 0.94 (0.59–1.48) | +/- | + | +++ | + | +/- | ? | +++ | + | ? | ||
| Ziprasidone | 0.72 (0.59–0.86) | - | ++ | + | - | - | ++ 0.41 (0.31–0.51) |
++/+ | + | ? | ||
| Zotepine | 0.69 (0.41–1.07) | + | +++ | ++ | +++/++ | +++/++ | ++ | +++ | ++ | Dose-dependent risk of seizures. Not licensed for use in the US. | ||
| Zuclopenthixol | ? | ++ | ++ | +++ | ++ | ++ | ? | +++ | + | Not licensed for use in the US. | ||
|
Note: "Notable" is to mean side-effects that are particularly unique to the antipsychotic drug in question. For example, clozapine is notorious for its ability to cause agranulocytosis. If data on the propensity of a particular drug to cause a particular AE is unavailable an estimation is substituted based on the pharmacologic profile of the drug.
| ||||||||||||
| Efficacy | |||||
|---|---|---|---|---|---|
| Generic drug name | Schizophrenia | Mania | Bipolar depression | Bipolar maintenance | Adjunct in major depression |
| Amisulpride | +++ | ? | ? | ? | ? (+++ in dysthymia) |
| Aripiprazole | ++ | ++ | - | ++ (prevents manic and mixed but not depressive episodes) | +++ |
| Asenapine | ++/+ | ++ | ? | ++ | ? |
| Chlorpromazine | ++ | ? | ? | ? | ? |
| Clozapine | +++ | +++ | +++ | +++ | +++ |
| Haloperidol | ++ | +++ | ? | ? | ? |
| Iloperidone | + | ? | ? | ? | ? |
| Loxapine | +++/++ | +++ (only in the treatment of agitation) | ? | ? | ? |
| Lurasidone | + | ? | +++ | ? | ? |
| Melperone | +++ | ? | ? | ? | ? |
| Olanzapine | +++ | +++/++ | ++ | ++ | ++ |
| Paliperidone | ++ | +++/++ | ? | ? | ? |
| Perospirone | + | ? | ? | ? | ? |
| Quetiapine | ++ | ++ | +++ | +++ | ++ |
| Risperidone | +++ | +++ | - | ++ | +++ |
| Sertindole | ++ | ? | ? | ? | ? |
| Ziprasidone | ++/+ | + | ? | + | ? |
| Zotepine | ++ | ? | ? | ? | ? |
| Binding affinity | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pharmacokinetics | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Medication | Brand name | Class | Vehicle | Dosage | Tmax | t1/2 single | t1/2 multiple | logP | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Aripiprazole lauroxil | Aristada | Atypical | Water | 441–1064 mg/4–8 weeks | 24–35 days | ? | 54–57 days | 7.9–10.0 | |
| Aripiprazole monohydrate | Abilify Maintena | Atypical | Water | 300–400 mg/4 weeks | 7 days | ? | 30–47 days | 4.9–5.2 | |
| Bromperidol decanoate | Impromen Decanoas | Typical | Sesame oil | 40–300 mg/4 weeks | 3–9 days | ? | 21–25 days | 7.9 | |
| Clopentixol decanoate | Sordinol Depot | Typical | Viscoleo | 50–600 mg/1–4 weeks | 4–7 days | ? | 19 days | 9.0 | |
| Flupentixol decanoate | Depixol | Typical | Viscoleo | 10–200 mg/2–4 weeks | 4–10 days | 8 days | 17 days | 7.2–9.2 | |
| Fluphenazine decanoate | Prolixin Decanoate | Typical | Sesame oil | 12.5–100 mg/2–5 weeks | 1–2 days | 1–10 days | 14–100 days | 7.2–9.0 | |
| Fluphenazine enanthate | Prolixin Enanthate | Typical | Sesame oil | 12.5–100 mg/1–4 weeks | 2–3 days | 4 days | ? | 6.4–7.4 | |
| Fluspirilene | Imap, Redeptin | Typical | Water | 2–12 mg/1 week | 1–8 days | 7 days | ? | 5.2–5.8 | |
| Haloperidol decanoate | Haldol Decanoate | Typical | Sesame oil | 20–400 mg/2–4 weeks | 3–9 days | 18–21 days | 7.2–7.9 | ||
| Olanzapine pamoate | Zyprexa Relprevv | Atypical | Water | 150–405 mg/2–4 weeks | 7 days | ? | 30 days | – | |
| Oxyprothepin decanoate | Meclopin | Typical | ? | ? | ? | ? | ? | 8.5–8.7 | |
| Paliperidone palmitate | Invega Sustenna | Atypical | Water | 39–819 mg/4–12 weeks | 13–33 days | 25–139 days | ? | 8.1–10.1 | |
| Perphenazine decanoate | Trilafon Dekanoat | Typical | Sesame oil | 50–200 mg/2–4 weeks | ? | ? | 27 days | 8.9 | |
| Perphenazine enanthate | Trilafon Enanthate | Typical | Sesame oil | 25–200 mg/2 weeks | 2–3 days | ? | 4–7 days | 6.4–7.2 | |
| Pipotiazine palmitate | Piportil Longum | Typical | Viscoleo | 25–400 mg/4 weeks | 9–10 days | ? | 14–21 days | 8.5–11.6 | |
| Pipotiazine undecylenate | Piportil Medium | Typical | Sesame oil | 100–200 mg/2 weeks | ? | ? | ? | 8.4 | |
| Risperidone | Risperdal Consta | Atypical | Microspheres | 12.5–75 mg/2 weeks | 21 days | ? | 3–6 days | – | |
| Zuclopentixol acetate | Clopixol Acuphase | Typical | Viscoleo | 50–200 mg/1–3 days | 1–2 days | 1–2 days | 4.7–4.9 | ||
| Zuclopentixol decanoate | Clopixol Depot | Typical | Viscoleo | 50–800 mg/2–4 weeks | 4–9 days | ? | 11–21 days | 7.5–9.0 | |
| Note: All by intramuscular injection. Footnotes: = Microcrystalline or nanocrystalline aqueous suspension. = Low-viscosity vegetable oil (specifically fractionated coconut oil with medium-chain triglycerides). = Predicted, from PubChem and DrugBank. Sources: Main: See template. | |||||||||
History


The original antipsychotic drugs were happened upon largely by chance and then tested for their effectiveness. The first, chlorpromazine, was developed as a surgical anesthetic. It was first used on psychiatric patients because of its powerful calming effect; at the time it was regarded as a non-permanent "pharmacological lobotomy". Lobotomy at the time was used to treat many behavioral disorders, including psychosis, although its effect was to markedly reduce behavior and mental functioning of all types. However, chlorpromazine proved to reduce the effects of psychosis in a more effective and specific manner than lobotomy, even though it was known to be capable of causing severe sedation. The underlying neurochemistry involved has since been studied in detail, and subsequent antipsychotic drugs have been developed by rational drug design.
The discovery of chlorpromazine's psychoactive effects in 1952 led to further research that resulted in the development of antidepressants, anxiolytics, and the majority of other drugs now used in the management of psychiatric conditions. In 1952, Henri Laborit described chlorpromazine only as inducing indifference towards what was happening around them in nonpsychotic, nonmanic patients, and Jean Delay and Pierre Deniker described it as controlling manic or psychotic agitation. The former claimed to have discovered a treatment for agitation in anyone, and the latter team claimed to have discovered a treatment for psychotic illness.
Until the 1970s there was considerable debate within psychiatry on the most appropriate term to use to describe the new drugs. In the late 1950s the most widely used term was "neuroleptic", followed by "major tranquilizer" and then "ataraxic". The first recorded use of the term tranquilizer dates from the early nineteenth century. In 1953 Frederik F. Yonkman, a chemist at the Swiss-based Cibapharmaceutical company, first used the term tranquilizer to differentiate reserpine from the older sedatives. The word neuroleptic was coined in 1955 by Delay and Deniker after their discovery (1952) of the antipsychotic effects of chlorpromazine. It is derived from the Greek: "νεῦρον" (neuron, originally meaning "sinew" but today referring to the nerves) and "λαμβάνω" (lambanō, meaning "take hold of"). Thus, the word means taking hold of one's nerves. It was often taken to refer also to common side effects such as reduced activity in general, as well as lethargy and impaired motor control. Although these effects are unpleasant and in some cases harmful, they were at one time, along with akathisia, considered a reliable sign that the drug was working. The term "ataraxy" was coined by the neurologist Howard Fabing and the classicist Alister Cameron to describe the observed effect of psychic indifference and detachment in patients treated with chlorpromazine. This term derived from the Greek adjective "ἀτάρακτος" (ataraktos), which means "not disturbed, not excited, without confusion, steady, calm". In the use of the terms "tranquilizer" and "ataractic", medical practitioners distinguished between the "major tranquilizers" or "major ataractics", which referred to drugs used to treat psychoses, and the "minor tranquilizers" or "minor ataractics", which referred to drugs used to treat neuroses. While popular during the 1950s, these terms are infrequently used today. They are being abandoned in favor of "antipsychotic", which refers to the drug's desired effects. Today, "minor tranquilizer" can refer to anxiolytic and/or hypnotic drugs such as the benzodiazepines and nonbenzodiazepines, which are useful as generally short-term management for insomnia together with cognitive behavioral therapy for insomnia. They are potentially addictive sedatives.
Antipsychotics are broadly divided into two groups, the typical or first-generation antipsychotics and the atypical or second-generation antipsychotics. The difference between first- and second-generation antipsychotics is a subject of debate. The second-generation antipsychotics are generally distinguishable by the presence of 5HT2A receptor antagonism and a corresponding lower propensity for extrapyramidal side effects compared to first-generation antipsychotics.
Society and culture
Terminology
The term major tranquilizer was used for older antipsychotic drugs. The term neuroleptic is often used as a synonym for antipsychotic, even though – strictly speaking – the two terms are not interchangeable. Antipsychotic drugs are a subgroup of neuroleptic drugs, because the latter have a wider range of effects.
Antipsychotics are a type of psychoactive or psychotropic medication.
Sales
Antipsychotics were once among the biggest selling and most profitable of all drugs, generating $22 billion in global sales in 2008. By 2003 in the US, an estimated 3.21 million patients received antipsychotics, worth an estimated $2.82 billion. Over 2/3 of prescriptions were for the newer, more expensive atypicals, each costing on average $164 per year, compared to $40 for the older types. By 2008, sales in the US reached $14.6 billion, the biggest selling drugs in the US by therapeutic class.
In the five years since July 2017 the number of antipsychotic medicines dispensed in the community in the United Kingdom has increased by 11.2%. There have also been substantial price rises. Risperidone 6 mg tablets, the largest, increased from £3.09 in July 2017 to £41.16 in June 2022. The NHS is spending an additional £33 million annually on antipsychotics. Haloperidol 500 microgram tablets constituted £14.3 million of this.
Overprescription
Antipsychotics in the nursing home population are often overprescribed, often for the purposes of making it easier to handle dementia patients. Federal efforts to reduce the use of antipsychotics in US nursing homes has led to a nationwide decrease in their usage in 2012.
Legal
Antipsychotics are sometimes administered as part of compulsory psychiatric treatment via inpatient (hospital) commitment or outpatient commitment.
Formulations
They may be administered orally or, in some cases, through long-acting (depot) injections administered in the dorsgluteal, ventrogluteal or deltoid muscle. Short-acting parenteral formulations also exist, which are generally reserved for emergencies or when oral administration is otherwise impossible. The oral formulations include immediate release, extended release, and orally disintegrating products (which are not sublingual, and can help ensure that medications are swallowed instead of "cheeked"). Sublingual products (e.g., asenapine) also exist, which must be held under the tongue for absorption. The first transdermal formulation of an antipsychotic (transdermal asenapine, marketed as Secuado), was FDA-approved in 2019.
Recreational use
Main article: Antipsychotics abuseCertain second-generation antipsychotics are misused or abused for their sedative, tranquilizing, and (paradoxically) "hallucinogenic" effects. The most commonly implicated second-generation antipsychotic is quetiapine. In case reports, quetiapine has been abused in doses taken by mouth (which is how the drug is available from the manufacturer), but also crushed and insufflated or mixed with water for injection into a vein. Olanzapine, another sedating second-generation antipsychotic, has also been misused for similar reasons. There is no standard treatment for antipsychotic abuse, though switching to a second-generation antipsychotic with less abuse potential (e.g., aripiprazole) has been used.
Controversy
Joanna Moncrieff has argued that antipsychotic drug treatment is often undertaken as a means of control rather than to treat specific symptoms experienced by the patient.
Use of this class of drugs has a history of criticism in residential care. As the drugs used can make patients calmer and more compliant, critics claim that the drugs can be overused. Outside doctors can feel under pressure from care home staff. In an official review commissioned by UK government ministers it was reported that the needless use of antipsychotic medication in dementia care was widespread and was linked to 1800 deaths per year. In the US, the government has initiated legal action against the pharmaceutical company Johnson & Johnson for allegedly paying kickbacks to Omnicare to promote its antipsychotic risperidone (Risperdal) in nursing homes.
There has also been controversy about the role of pharmaceutical companies in marketing and promoting antipsychotics, including allegations of downplaying or covering up adverse effects, expanding the number of conditions or illegally promoting off-label usage; influencing drug trials (or their publication) to try to show that the expensive and profitable newer atypicals were superior to the older cheaper typicals that were out of patent. Following charges of illegal marketing, settlements by two large pharmaceutical companies in the US set records for the largest criminal fines ever imposed on corporations. One case involved Eli Lilly and Company's antipsychotic Zyprexa, and the other involved Bextra. In the Bextra case, the government also charged Pfizer with illegally marketing another antipsychotic, Geodon. In addition, AstraZeneca faces numerous personal-injury lawsuits from former users of Seroquel (quetiapine), amidst federal investigations of its marketing practices. By expanding the conditions for which they were indicated, Astrazeneca's Seroquel and Eli Lilly's Zyprexa had become the biggest selling antipsychotics in 2008 with global sales of $5.5 billion and $5.4 billion respectively.
Harvard University medical professor Joseph Biederman conducted research on bipolar disorder in children that led to an increase in such diagnoses. A 2008 Senate investigation found that Biederman also received $1.6 million in speaking and consulting fees between 2000 and 2007, some of them undisclosed to Harvard, from companies including makers of antipsychotic drugs prescribed for children with bipolar disorder. Johnson & Johnson gave more than $700,000 to a research center that was headed by Biederman from 2002 to 2005, where research was conducted, in part, on Risperdal, the company's antipsychotic drug. Biederman has responded saying that the money did not influence him and that he did not promote a specific diagnosis or treatment.
Pharmaceutical companies have also been accused of attempting to set the mental health agenda through activities such as funding consumer advocacy groups.
Special populations
It is recommended that persons with dementia who exhibit behavioral and psychological symptoms should not be given antipsychotics before trying other treatments. When taking antipsychotics this population has increased risk of cerebrovascular effects, parkinsonism or extrapyramidal symptoms, sedation, confusion and other cognitive adverse effects, weight gain, and increased mortality. Physicians and caretakers of persons with dementia should try to address symptoms including agitation, aggression, apathy, anxiety, depression, irritability, and psychosis with alternative treatments whenever antipsychotic use can be replaced or reduced. Elderly persons often have their dementia treated first with antipsychotics and this is not the best management strategy.
See also
Notes
- Bolded drug names indicate drugs that are metabolites of clinically-marketed antipsychotics.
References
- ^ Finkel R, Clark MA, Cubeddu LX (2009). Pharmacology. Lippincott Williams & Wilkins. p. 151. ISBN 978-0-7817-7155-9. Archived from the original on 1 April 2017.
- Burnett GB (1975). "The assessment of thiothixene in chronic schizophrenia. A double-blind controlled trial". Dis Nerv Syst. 36 (11): 625–9. PMID 1102277.
- ^ Bartoli F, Cavaleri D, Callovini T, Riboldi I, Crocamo C, D'Agostino A, Martinotti G, Bertolini F, Ostuzzi G, Barbui C, Carrà G (March 2022). "Comparing 1-year effectiveness and acceptability of once-monthly paliperidone palmitate and aripiprazole monohydrate for schizophrenia spectrum disorders: Findings from the STAR Network Depot Study". Psychiatry Research. 309: 114405. doi:10.1016/j.psychres.2022.114405. PMID 35093701. S2CID 246054926.
- Lally J, MacCabe JH (June 2015). "Antipsychotic medication in schizophrenia: a review". British Medical Bulletin. 114 (1): 169–79. doi:10.1093/bmb/ldv017. PMID 25957394.
Antipsychotic medications are mainstays in the treatment of schizophrenia and a range of other psychotic disorders.
- Grande I, Berk M, Birmaher B, Vieta E (April 2016). "Bipolar disorder". Lancet. 387 (10027): 1561–1572. doi:10.1016/S0140-6736(15)00241-X. PMID 26388529. S2CID 205976059.
- ^ Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V (February 2011). "Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia". Archives of General Psychiatry. 68 (2): 128–137. doi:10.1001/archgenpsychiatry.2010.199. PMC 3476840. PMID 21300943.
- ^ Moncrieff J, Leo J (September 2010). "A systematic review of the effects of antipsychotic drugs on brain volume". Psychological Medicine. 40 (9): 1409–1422. doi:10.1017/S0033291709992297. PMID 20085668. S2CID 23522488.
- ^ Chopra S, Fornito A, Francey SM, O'Donoghue B, Cropley V, Nelson B, et al. (July 2021). "Differentiating the effect of antipsychotic medication and illness on brain volume reductions in first-episode psychosis: A Longitudinal, Randomised, Triple-blind, Placebo-controlled MRI Study". Neuropsychopharmacology. 46 (8): 1494–1501. doi:10.1038/s41386-021-00980-0. PMC 8209146. PMID 33637835.
- please see Chopra et al 2021: Strengths and limitations "only examined risperidone and paliperidone"
- please see Chopra et al 2021: Method Study design
- please see Chopra et al 2021: Introduction, 3rd paragraph, Lieberman JA, et al. 2005 & Shao Y et al 2015, and Chopra et al: Are antipsychotics neuroprotective? 1st paragraph last sentence
- Shen WW (December 1999). "A history of antipsychotic drug development". Comprehensive Psychiatry. 40 (6): 407–14. doi:10.1016/s0010-440x(99)90082-2. PMID 10579370.
- Aringhieri S, Carli M, Kolachalam S, Verdesca V, Cini E, Rossi M, et al. (December 2018). "Molecular targets of atypical antipsychotics: From mechanism of action to clinical differences". Pharmacology & Therapeutics. 192: 20–41. doi:10.1016/j.pharmthera.2018.06.012. PMID 29953902. S2CID 49602956.
- ^ Mailman RB, Murthy V (2010). "Third generation antipsychotic drugs: partial agonism or receptor functional selectivity?". Current Pharmaceutical Design. 16 (5): 488–501. doi:10.2174/138161210790361461. PMC 2958217. PMID 19909227.
- ^ King C, Voruganti LN (May 2002). "What's in a name? The evolution of the nomenclature of antipsychotic drugs". Journal of Psychiatry & Neuroscience. 27 (3): 168–175. PMC 161646. PMID 12066446.
- ^ Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM (January 2009). "Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis". Lancet. 373 (9657): 31–41. doi:10.1016/S0140-6736(08)61764-X. PMID 19058842. S2CID 1071537.
- Goikolea JM, Colom F, Torres I, Capapey J, Valentí M, Undurraga J, Grande I, Sanchez-Moreno J, Vieta E (January 2013). "Lower rate of depressive switch following antimanic treatment with second-generation antipsychotics versus haloperidol". Journal of Affective Disorders. 144 (3): 191–8. doi:10.1016/j.jad.2012.07.038. PMID 23089129.
- ^ Taylor D, Paton C, Kapur S, Taylor D (2012). The Maudsley prescribing guidelines in psychiatry (11th ed.). Chichester, West Sussex, UK: Wiley-Blackwell. ISBN 978-0-470-97948-8.
- ^ "American Psychiatric Association Five Things Physicians and Patients Should Question". Choosing Wisely. Archived from the original on 3 December 2013. Retrieved 23 September 2013.
- Budman CL (July 2014). "The role of atypical antipsychotics for treatment of Tourette's syndrome: an overview". Drugs. 74 (11): 1177–1193. doi:10.1007/s40265-014-0254-0. PMID 25034359. S2CID 24378317.
- Basson R, Gilks T (1 January 2018). "Women's sexual dysfunction associated with psychiatric disorders and their treatment". Women's Health. 14: 1745506518762664. doi:10.1177/1745506518762664. PMC 5900810. PMID 29649948.
- Kreys TJ, Phan SV (February 2015). "A literature review of quetiapine for generalized anxiety disorder". Pharmacotherapy. 35 (2): 175–188. doi:10.1002/phar.1529. PMID 25689246. S2CID 24744675.
- ^ "Psychosis and schizophrenia in adults (CG178)". 12 February 2014. Archived from the original on 4 March 2014.
- "American Psychiatric Association Practice Guidelines". Psychiatry Online.
- ^ Barnes TR (May 2011). "Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology". Journal of Psychopharmacology. 25 (5): 567–620. doi:10.1177/0269881110391123. PMID 21292923. S2CID 40089561.
- Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW, Lieberman JA (December 2012). "Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents". Molecular Psychiatry. 17 (12): 1206–27. doi:10.1038/mp.2012.47. PMID 22584864.
- ^ Hartling L, Abou-Setta AM, Dursun S, Mousavi SS, Pasichnyk D, Newton AS (October 2012). "Antipsychotics in adults with schizophrenia: comparative effectiveness of first-generation versus second-generation medications: a systematic review and meta-analysis". Annals of Internal Medicine. 157 (7): 498–511. doi:10.7326/0003-4819-157-7-201210020-00525. PMID 22893011.
- Furukawa TA, Levine SZ, Tanaka S, Goldberg Y, Samara M, Davis JM, Cipriani A, Leucht S (January 2015). "Initial severity of schizophrenia and efficacy of antipsychotics: participant-level meta-analysis of 6 placebo-controlled studies". JAMA Psychiatry. 72 (1): 14–21. doi:10.1001/jamapsychiatry.2014.2127. PMID 25372935.
- Keefe RS, Silva SG, Perkins DO, Lieberman JA (1 January 1999). "The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis". Schizophrenia Bulletin. 25 (2): 201–22. doi:10.1093/oxfordjournals.schbul.a033374. PMID 10416727.
- Brodeur S, Chiu YM, Courteau J, Dorais M, Oliver D, Stip E, Fleury MJ, Roy MA, Vanasse A, Lesage A, Leclerc J (22 November 2024). "Medication Exposure and Mortality in Patients With Schizophrenia". JAMA Network Open. 7 (11): e2447137. doi:10.1001/jamanetworkopen.2024.47137. ISSN 2574-3805. PMC 11584925. PMID 39576638.
- "Can Antipsychotics Increase Mortality Risk? A New Study Shows It Might". HCP Live. 26 November 2024. Retrieved 29 November 2024.
- "NICE Treatment Guidance 2014". Archived from the original on 13 August 2014. Retrieved 7 August 2014.
- McGorry PD, Hartmann JA, Spooner R, Nelson B (June 2018). "Beyond the "at risk mental state" concept: transitioning to transdiagnostic psychiatry". World Psychiatry. 17 (2): 133–142. doi:10.1002/wps.20514. PMC 5980504. PMID 29856558.
- ^ Starzer MS, Nordentoft M, Hjorthøj C (April 2018). "Rates and Predictors of Conversion to Schizophrenia or Bipolar Disorder Following Substance-Induced Psychosis". The American Journal of Psychiatry. 175 (4). American Psychiatric Association Publishing: 343–350. doi:10.1176/appi.ajp.2017.17020223. PMID 29179576.
- Robinson DG, Gallego JA, John M, Petrides G, Hassoun Y, Zhang JP, et al. (November 2015). "A Randomized Comparison of Aripiprazole and Risperidone for the Acute Treatment of First-Episode Schizophrenia and Related Disorders: 3-Month Outcomes". Schizophrenia Bulletin. 41 (6): 1227–1236. doi:10.1093/schbul/sbv125. PMC 4601722. PMID 26338693.
- Gómez-Revuelta M, Pelayo-Terán JM, Juncal-Ruiz M, Vázquez-Bourgon J, Suárez-Pinilla P, Romero-Jiménez R, et al. (April 2020). "Antipsychotic Treatment Effectiveness in First Episode of Psychosis: PAFIP 3-Year Follow-Up Randomized Clinical Trials Comparing Haloperidol, Olanzapine, Risperidone, Aripiprazole, Quetiapine, and Ziprasidone". The International Journal of Neuropsychopharmacology. 23 (4): 217–229. doi:10.1093/ijnp/pyaa004. PMC 7177160. PMID 31974576.
- Leucht S, Arbter D, Engel RR, Kissling W, Davis JM (April 2009). "How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials". Molecular Psychiatry. 14 (4): 429–47. doi:10.1038/sj.mp.4002136. PMID 18180760.
- ^ Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lässig B, Salanti G, Davis JM (September 2013). "Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis". Lancet. 382 (9896): 951–62. doi:10.1016/S0140-6736(13)60733-3. PMID 23810019. S2CID 32085212.
- Beitinger R, Lin J, Kissling W, Leucht S (October 2008). "Comparative remission rates of schizophrenic patients using various remission criteria". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 32 (7): 1643–51. doi:10.1016/j.pnpbp.2008.06.008. PMID 18616969. S2CID 207408308.
- ^ Ceraso A, Lin JJ, Schneider-Thoma J, Siafis S, Tardy M, Komossa K, et al. (August 2020). "Maintenance treatment with antipsychotic drugs for schizophrenia". The Cochrane Database of Systematic Reviews. 2020 (8): CD008016. doi:10.1002/14651858.CD008016.pub3. PMC 9702459. PMID 32840872. S2CID 221306099.
- ^ Tiihonen J, Tanskanen A, Taipale H (August 2018). "20-Year Nationwide Follow-Up Study on Discontinuation of Antipsychotic Treatment in First-Episode Schizophrenia". The American Journal of Psychiatry. 175 (8). American Psychiatric Association Publishing: 765–773. doi:10.1176/appi.ajp.2018.17091001. PMID 29621900.
- Kinon BJ, Ascher-Svanum H, Adams DH, Chen L (October 2008). "The temporal relationship between symptom change and treatment discontinuation in a pooled analysis of 4 schizophrenia trials". Journal of Clinical Psychopharmacology. 28 (5): 544–9. doi:10.1097/JCP.0b013e318185e74a. PMID 18794651. S2CID 203910.
- ^ Park SC, Choi MY, Choi J, Park E, Tchoe HJ, Suh JK, et al. (November 2018). "Comparative Efficacy and Safety of Long-acting Injectable and Oral Second-generation Antipsychotics for the Treatment of Schizophrenia: A Systematic Review and Meta-analysis". Clinical Psychopharmacology and Neuroscience. 16 (4): 361–375. doi:10.9758/cpn.2018.16.4.361. PMC 6245299. PMID 30466208.
- "PSYCHOSIS and Schizophreniain adults: THE NICE GUIDELINE ON TREATMENT AND MANAGEMENT". p. 10.11.1.27.
- Lambert TJ, Singh BS, Patel MX (November 2009). "Community treatment orders and antipsychotic long-acting injections". The British Journal of Psychiatry. Supplement. 52 (S52). Royal College of Psychiatrists: S57-62. doi:10.1192/bjp.195.52.s57. PMID 19880919.
- ^ Young LL, Kradjan WA, Guglielmo BJ, Corelli RL, Williams BR, Koda-Kimble MA (2009). Applied therapeutics: the clinical use of drugs (9th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 3040. ISBN 978-0-7817-6555-8.
- Correll CU, Sheridan EM, DelBello MP (March 2010). "Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials". Bipolar Disorders. 12 (2): 116–41. doi:10.1111/j.1399-5618.2010.00798.x. PMID 20402706.
- "DailyMed - CAPLYTA- lumateperone capsule". dailymed.nlm.nih.gov. Retrieved 10 December 2022.
- Earley W, Burgess MV, Rekeda L, Dickinson R, Szatmári B, Németh G, et al. (June 2019). "Cariprazine Treatment of Bipolar Depression: A Randomized Double-Blind Placebo-Controlled Phase 3 Study". The American Journal of Psychiatry. 176 (6): 439–448. doi:10.1176/appi.ajp.2018.18070824. PMID 30845817. S2CID 73471270.
- Lowes R. "Lurasidone Approved for Bipolar Depression". Medscape. Archived from the original on 2 October 2013. Retrieved 2 October 2013.
- Tohen M, Katagiri H, Fujikoshi S, Kanba S (July 2013). "Efficacy of olanzapine monotherapy in acute bipolar depression: a pooled analysis of controlled studies". Journal of Affective Disorders. 149 (1–3): 196–201. doi:10.1016/j.jad.2013.01.022. PMID 23485111.
- Thase ME (February 2008). "Quetiapine monotherapy for bipolar depression". Neuropsychiatric Disease and Treatment. 4 (1): 11–21. doi:10.2147/ndt.s1162. PMC 2515925. PMID 18728771.
- Tohen M, Greil W, Calabrese JR, Sachs GS, Yatham LN, Oerlinghausen BM, et al. (July 2005). "Olanzapine versus lithium in the maintenance treatment of bipolar disorder: a 12-month, randomized, double-blind, controlled clinical trial". The American Journal of Psychiatry. 162 (7): 1281–1290. doi:10.1176/appi.ajp.162.7.1281. PMID 15994710. S2CID 20932562.
- Duffy A, Milin R, Grof P (February 2009). "Maintenance treatment of adolescent bipolar disorder: open study of the effectiveness and tolerability of quetiapine". BMC Psychiatry. 9: 4. doi:10.1186/1471-244X-9-4. PMC 2644292. PMID 19200370.
- Weisler RH, Nolen WA, Neijber A, Hellqvist A, Paulsson B (November 2011). "Continuation of quetiapine versus switching to placebo or lithium for maintenance treatment of bipolar I disorder (Trial 144: a randomized controlled study)". The Journal of Clinical Psychiatry. 72 (11): 1452–1464. doi:10.4088/JCP.11m06878. PMID 22054050.
- Cipriani A, Rendell JM, Geddes J (January 2009). Cipriani A (ed.). "Olanzapine in long-term treatment for bipolar disorder". The Cochrane Database of Systematic Reviews (1): CD004367. doi:10.1002/14651858.CD004367.pub2. PMID 19160237. S2CID 205173641.
- Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, Kreyenbuhl J (February 2004). "Practice guideline for the treatment of patients with schizophrenia, second edition". The American Journal of Psychiatry. 161 (2 Suppl): 1–56. PMID 15000267.
- National Collaborating Centre (2009). "Schizophrenia". NCBI Bookshelf. PMID 20704054. Retrieved 9 March 2021.
- ^ Ventimiglia J, Kalali AH, Vahia IV, Jeste DV (November 2010). "An analysis of the intended use of atypical antipsychotics in dementia". Psychiatry. 7 (11): 14–17. PMC 3010964. PMID 21191528.
- AMDA – The Society for Post-Acute and Long-Term Care Medicine (February 2014). "Ten Things Physicians and Patients Should Question". Choosing Wisely: an initiative of the ABIM Foundation. AMDA – The Society for Post-Acute and Long-Term Care Medicine. Archived from the original on 13 September 2014. Retrieved 20 April 2015..
- Ballard C, Waite J (January 2006). Ballard CG (ed.). "The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer's disease". The Cochrane Database of Systematic Reviews (1): CD003476. doi:10.1002/14651858.CD003476.pub2. PMC 11365591. PMID 16437455.
- Lühnen J, Richter T, Calo S, Meyer G, Köpke S, Möhler R (August 2023). "Psychosocial interventions for reducing antipsychotic medication in care home residents". The Cochrane Database of Systematic Reviews. 2023 (8): CD008634. doi:10.1002/14651858.CD008634.pub3. PMC 10471006. PMID 37650479.
- ^ Komossa K, Depping AM, Gaudchau A, Kissling W, Leucht S (December 2010). "Second-generation antipsychotics for major depressive disorder and dysthymia". The Cochrane Database of Systematic Reviews (12): CD008121. doi:10.1002/14651858.CD008121.pub2. PMID 21154393.
- Spielmans GI, Berman MI, Linardatos E, Rosenlicht NZ, Perry A, Tsai AC (2013). "Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes". PLOS Medicine. 10 (3): e1001403. doi:10.1371/journal.pmed.1001403. PMC 3595214. PMID 23554581.
- Truven Health Analytics, Inc. DrugPoint System (Internet) . Greenwood Village, CO: Thomsen Healthcare; 2013.
- "FDA Psychopharmacologic Drugs Advisory Committee Hearing" (PDF). American Academy of Child & Adolescent Psychiatry. Archived (PDF) from the original on 9 October 2022. Retrieved 31 May 2020.
- Al-Wandi A, Landén M, Nordenskjöld A (6 November 2023). "Antipsychotics in the maintenance phase for psychotic depression". Acta Psychiatrica Scandinavica. 149 (1): 6–17. doi:10.1111/acps.13628. ISSN 0001-690X. PMID 37932158.
- Hálfdánarson Ó, Zoëga H, Aagaard L, Bernardo M, Brandt L, Fusté AC, Furu K, Garuoliené K, Hoffmann F, Huybrechts KF, Kalverdijk LJ, Kawakami K, Kieler H, Kinoshita T, Litchfield M (October 2017). "International trends in antipsychotic use: A study in 16 countries, 2005-2014". European Neuropsychopharmacology. 27 (10): 1064–1076. doi:10.1016/j.euroneuro.2017.07.001. hdl:1959.4/unsworks_79133. ISSN 1873-7862. PMID 28755801.
- Radha Krishnan RP, Harrison C, Buckley N, Raubenheimer JE (April 2024). "On- and off-label utilisation of antipsychotics in Australia (2000–2021): Retrospective analysis of two medication datasets". Australian & New Zealand Journal of Psychiatry. 58 (4): 320–333. doi:10.1177/00048674231210209. ISSN 0004-8674. PMC 10960313. PMID 37941354.
- McKean A, Monasterio E (1 May 2012). "Off-label use of atypical antipsychotics: cause for concern?". CNS Drugs. 26 (5): 383–390. doi:10.2165/11632030-000000000-00000. ISSN 1179-1934. PMID 22448598.
- ^ Maher AR, Theodore G (June 2012). "Summary of the comparative effectiveness review on off-label use of atypical antipsychotics". Journal of Managed Care Pharmacy. 18 (5 Suppl B): S1-20. doi:10.18553/jmcp.2012.18.s5-b.1. PMC 10438344. PMID 22784311.
- ^ Maglione M, Maher AR, Hu J, Wang Z, Shanman R, Shekelle PG, Roth B, Hilton L, Suttorp MJ (2011). Off-Label Use of Atypical Antipsychotics: An Update. Comparative Effectiveness Reviews, No. 43. Rockville: Agency for Healthcare Research and Quality. PMID 22973576.
- Coe HV, Hong IS (May 2012). "Safety of low doses of quetiapine when used for insomnia". The Annals of Pharmacotherapy. 46 (5): 718–722. doi:10.1345/aph.1Q697. PMID 22510671. S2CID 9888209.
- Pillinger T, McCutcheon RA, Vano L, Mizuno Y, Arumuham A, Hindley G, et al. (January 2020). "Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis". The Lancet. Psychiatry. 7 (1): 64–77. doi:10.1016/s2215-0366(19)30416-x. PMC 7029416. PMID 31860457.
- Yoshida K, Takeuchi H (March 2021). "Dose-dependent effects of antipsychotics on efficacy and adverse effects in schizophrenia". Behavioural Brain Research. 402: 113098. doi:10.1016/j.bbr.2020.113098. PMID 33417992. S2CID 230507941.
- De Crescenzo F, D'Alò GL, Ostinelli EG, Ciabattini M, Di Franco V, Watanabe N, et al. (July 2022). "Comparative effects of pharmacological interventions for the acute and long-term management of insomnia disorder in adults: a systematic review and network meta-analysis". Lancet. 400 (10347): 170–184. doi:10.1016/S0140-6736(22)00878-9. hdl:11380/1288245. PMID 35843245. S2CID 250536370.
- Work Group on Borderline Personality Disorder (2001). Practice Guideline for the Treatment of Patients With Borderline Personality Disorder. American Psychiatric Association Publications. p. 4. ISBN 978-0-89042-319-6. Retrieved 5 June 2013.
- "Antipsychotics are commonly prescribed to people with personality disorders, contrary to guidelines". NIHR Evidence. 10 November 2022. doi:10.3310/nihrevidence_54520. S2CID 253467990.
- Hardoon S, Hayes J, Viding E, McCrory E, Walters K, Osborn D (March 2022). "Prescribing of antipsychotics among people with recorded personality disorder in primary care: a retrospective nationwide cohort study using The Health Improvement Network primary care database". BMJ Open. 12 (3): e053943. doi:10.1136/bmjopen-2021-053943. PMC 8968526. PMID 35264346.
- Zuddas A, Zanni R, Usala T (August 2011). "Second generation antipsychotics (SGAs) for non-psychotic disorders in children and adolescents: a review of the randomized controlled studies". European Neuropsychopharmacology. 21 (8): 600–20. doi:10.1016/j.euroneuro.2011.04.001. PMID 21550212. S2CID 1254352.
- Pringsheim T, Doja A, Gorman D, McKinlay D, Day L, Billinghurst L, Carroll A, Dion Y, Luscombe S, Steeves T, Sandor P (March 2012). "Canadian guidelines for the evidence-based treatment of tic disorders: pharmacotherapy". Canadian Journal of Psychiatry. 57 (3): 133–43. doi:10.1177/070674371205700302. PMID 22397999.
- McPheeters ML, Warren Z, Sathe N, Bruzek JL, Krishnaswami S, Jerome RN, Veenstra-Vanderweele J (May 2011). "A systematic review of medical treatments for children with autism spectrum disorders". Pediatrics. 127 (5): e1312–21. doi:10.1542/peds.2011-0427. PMID 21464191. S2CID 2903864.
- "Evidence Lacking to Support Many Off-label Uses of Atypical Antipsychotics" (Press release). Agency for Healthcare Research and Quality. 17 January 2007. Archived from the original on 25 February 2013. Retrieved 29 July 2013.
- James AC (2010). "Prescribing antipsychotics for children and adolescents". Advances in Psychiatric Treatment. 16 (1): 63–75. doi:10.1192/apt.bp.108.005652.
- Truven Health Analytics, Inc. DRUGDEX System (Internet) . Greenwood Village, CO: Thomsen Healthcare; 2013.
- "Antipsychotics are increasingly prescribed to children and teenagers". NIHR Evidence. 18 April 2023. doi:10.3310/nihrevidence_57289. S2CID 258224542.
- Radojčić MR, Pierce M, Hope H, Senior M, Taxiarchi VP, Trefan L, et al. (February 2023). "Trends in antipsychotic prescribing to children and adolescents in England: cohort study using 2000-19 primary care data". The Lancet. Psychiatry. 10 (2): 119–128. doi:10.1016/s2215-0366(22)00404-7. PMID 36638816. S2CID 255703855.
- Romeo R, Knapp M, Tyrer P, Crawford M, Oliver-Africano P (July 2009). "The treatment of challenging behaviour in intellectual disabilities: cost-effectiveness analysis". Journal of Intellectual Disability Research. 53 (7): 633–43. doi:10.1111/j.1365-2788.2009.01180.x. PMID 19460067. S2CID 34448894.
- Linton D, Barr AM, Honer WG, Procyshyn RM (May 2013). "Antipsychotic and psychostimulant drug combination therapy in attention deficit/hyperactivity and disruptive behavior disorders: a systematic review of efficacy and tolerability". Current Psychiatry Reports. 15 (5): 355. doi:10.1007/s11920-013-0355-6. PMID 23539465. S2CID 45484062.
- Oh ES, Needham DM, Nikooie R, Wilson LM, Zhang A, Robinson KA, Neufeld KJ (October 2019). "Antipsychotics for Preventing Delirium in Hospitalized Adults: A Systematic Review". Annals of Internal Medicine. 171 (7): 474–484. doi:10.7326/M19-1859. PMID 31476766.
- Kane JM, Correll CU (2010). "Pharmacologic treatment of schizophrenia". Dialogues in Clinical Neuroscience. 12 (3): 345–57. doi:10.31887/DCNS.2010.12.3/jkane. PMC 3085113. PMID 20954430.
- Barry SJ, Gaughan TM, Hunter R (June 2012). "Schizophrenia". BMJ Clinical Evidence. 2012. PMC 3385413. PMID 23870705.
- Schultz SH, North SW, Shields CG (June 2007). "Schizophrenia: a review". American Family Physician. 75 (12): 1821–9. CiteSeerX 10.1.1.602.7571. PMID 17619525.
- Taylor DM, Duncan-McConnell D (2000). "Refractory schizophrenia and atypical antipsychotics". Journal of Psychopharmacology. 14 (4): 409–18. doi:10.1177/026988110001400411. PMID 11198061. S2CID 27270415.
- Essali A, Al-Haj Haasan N, Li C, Rathbone J (January 2009). "Clozapine versus typical neuroleptic medication for schizophrenia". The Cochrane Database of Systematic Reviews. 2009 (1): CD000059. doi:10.1002/14651858.CD000059.pub2. PMC 7065592. PMID 19160174.
- Heres S, Davis J, Maino K, Jetzinger E, Kissling W, Leucht S (February 2006). "Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine: an exploratory analysis of head-to-head comparison studies of second-generation antipsychotics". The American Journal of Psychiatry. 163 (2): 185–94. doi:10.1176/appi.ajp.163.2.185. PMID 16449469. S2CID 3849348.
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK (September 2005). "Effectiveness of antipsychotic drugs in patients with chronic schizophrenia". The New England Journal of Medicine. 353 (12): 1209–23. doi:10.1056/NEJMoa051688. PMID 16172203. S2CID 22499842.
- Swartz MS, Stroup TS, McEvoy JP, Davis SM, Rosenheck RA, Keefe RS, Hsiao JK, Lieberman JA (May 2008). "Special Section on Implications of CATIE: What CATIE Found: Results From the Schizophrenia Trial". Psychiatric Services. 59 (5): 500–506. doi:10.1176/ps.2008.59.5.500. ISSN 1075-2730. PMC 5033643. PMID 18451005.
- Voruganti LP, Baker LK, Awad AG (March 2008). "New generation antipsychotic drugs and compliance behaviour". Current Opinion in Psychiatry. 21 (2): 133–9. doi:10.1097/YCO.0b013e3282f52851. PMID 18332660. S2CID 34935.
- Paczynski RP, Alexander GC, Chinchilli VM, Kruszewski SP (January 2012). "Quality of evidence in drug compendia supporting off-label use of typical and atypical antipsychotic medications". The International Journal of Risk & Safety in Medicine. 24 (3): 137–146. doi:10.3233/JRS-2012-0567. PMID 22936056.
- Owens DC (2008). "How CATIE brought us back to Kansas: a critical re-evaluation of the concept of atypical antipsychotics and their place in the treatment of schizophrenia". Advances in Psychiatric Treatment. 14 (1): 17–28. doi:10.1192/apt.bp.107.003970.
- Fischer-Barnicol D, Lanquillon S, Haen E, Zofel P, Koch HJ, Dose M, Klein HE (2008). "Typical and atypical antipsychotics--the misleading dichotomy. Results from the Working Group 'Drugs in Psychiatry' (AGATE)". Neuropsychobiology. 57 (1–2): 80–87. doi:10.1159/000135641. PMID 18515977. S2CID 2669203.
- Casey DE (March 1999). "Tardive dyskinesia and atypical antipsychotic drugs". Schizophrenia Research. 35 (Suppl 1): S61–S66. doi:10.1016/S0920-9964(98)00160-1. PMID 10190226. S2CID 31807817.
- Makhinson M (January 2010). "Biases in medication prescribing: the case of second-generation antipsychotics". Journal of Psychiatric Practice. 16 (1): 15–21. doi:10.1097/01.pra.0000367774.11260.e4. PMID 20098227. S2CID 46530288.
- Halman A, Kong G, Sarris J, Perkins D (January 2024). "Drug-drug interactions involving classic psychedelics: A systematic review". J Psychopharmacol. 38 (1): 3–18. doi:10.1177/02698811231211219. PMC 10851641. PMID 37982394.
- Yates G, Melon E (January 2024). "Trip-killers: a concerning practice associated with psychedelic drug use". Emerg Med J. 41 (2): 112–113. doi:10.1136/emermed-2023-213377. PMID 38123961.
- Suran M (February 2024). "Study Finds Hundreds of Reddit Posts on "Trip-Killers" for Psychedelic Drugs". JAMA. 331 (8): 632–634. doi:10.1001/jama.2023.28257. PMID 38294772.
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D (December 1998). "Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action". NeuroReport. 9 (17): 3897–3902. doi:10.1097/00001756-199812010-00024. PMID 9875725.
- ^ American Psychiatric Association (September 2013). "Five Things Physicians and Patients Should Question". Choosing Wisely: an initiative of the ABIM Foundation. American Psychiatric Association. Archived from the original on 3 December 2013. Retrieved 30 December 2013., which cites
- Practice Guideline for the Treatment of Patients With Schizophrenia. Vol. 1 (Second ed.). American Psychiatric Association. 2006. doi:10.1176/appi.books.9780890423363.45859 (inactive 4 December 2024). ISBN 978-0-89042-336-3.
{{cite book}}: CS1 maint: DOI inactive as of December 2024 (link) - Joint Commission (30 June 2013). "HBIPS-4, Patients discharged on multiple antipsychotic medications". Specifications Manual for Joint Commission National Quality Core Measures. Archived from the original on 10 November 2013. Retrieved 27 October 2013.
- Stahl SM, Grady MM (February 2004). "A critical review of atypical antipsychotic utilization: comparing monotherapy with polypharmacy and augmentation". Current Medicinal Chemistry. 11 (3): 313–27. doi:10.2174/0929867043456070. PMID 14965234.
- Practice Guideline for the Treatment of Patients With Schizophrenia. Vol. 1 (Second ed.). American Psychiatric Association. 2006. doi:10.1176/appi.books.9780890423363.45859 (inactive 4 December 2024). ISBN 978-0-89042-336-3.
- ^ Barry SJ, Gaughan TM, Hunter R (June 2012). "Schizophrenia". BMJ Clin Evid. 2012. PMC 3385413. PMID 23870705.
- Nakata Y, Kanahara N, Iyo M (December 2017). "Dopamine supersensitivity psychosis in schizophrenia: Concepts and implications in clinical practice". Journal of Psychopharmacology. 31 (12): 1511–1518. doi:10.1177/0269881117728428. PMID 28925317. S2CID 1957881.
- Ware MR, Feller DB, Hall KL (4 January 2018). "Neuroleptic Malignant Syndrome: Diagnosis and Management". The Primary Care Companion for CNS Disorders. 20 (1). doi:10.4088/PCC.17r02185. PMID 29325237.
- Carbon M, Kane JM, Leucht S, Correll CU (October 2018). "Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis". World Psychiatry. 17 (3): 330–340. doi:10.1002/wps.20579. PMC 6127753. PMID 30192088.
- Moore DP, Puri BK (2012). Textbook of Clinical Neuropsychiatry and Behavioral Neuroscience, Third Edition. CRC Press. p. 791. ISBN 978-1-4441-6494-7. Archived from the original on 25 November 2017.
- ^ De Berardis D, Rapini G, Olivieri L, et al. (May 2018). "Safety of antipsychotics for the treatment of schizophrenia: a focus on the adverse effects of clozapine". Therapeutic Advances in Drug Safety. 9 (5): 237–256. doi:10.1177/2042098618756261. PMC 5956953. PMID 29796248.
- Legge SE, Walters JT (March 2019). "Genetics of clozapine-associated neutropenia: recent advances, challenges and future perspective". Pharmacogenomics. 20 (4): 279–290. doi:10.2217/pgs-2018-0188. PMC 6563116. PMID 30767710.
- Kritharides L, Chow V, Lambert TJ (6 February 2017). "Cardiovascular disease in patients with schizophrenia". The Medical Journal of Australia. 206 (2): 91–95. doi:10.5694/mja16.00650. PMID 28152356. S2CID 5388097.
- ^ Sarvaiya N, Lapitskaya Y, Dima L, Manu P (August 2018). "Clozapine-Associated Pulmonary Embolism: A High-Mortality, Dose-Independent and Early-Onset Adverse Effect". American Journal of Therapeutics. 25 (4): e434–e438. doi:10.1097/MJT.0000000000000806. PMID 29985823. S2CID 51608744.
- Teodoro T, Nogueira V, Aldeias J, Teles Martins M, Salgado J (September 2022). "Clozapine Associated Periorbital Edema in First Episode Psychosis: A Case Report of a Rare Adverse Effect in Treatment-Resistant Schizophrenia". Journal of Clinical Psychopharmacology. 42 (6): 594–596. doi:10.1097/JCP.0000000000001600. PMID 36066404. S2CID 252088054.
- Citrome L, McEvoy JP, Saklad SR (2016). "Guide to the Management of Clozapine-Related Tolerability and Safety Concerns". Clinical Schizophrenia & Related Psychoses. 10 (3): 163–177. doi:10.3371/1935-1232.10.3.163. PMID 27732102.
- Sriretnakumar V, Huang E, Müller DJ (2015). "Pharmacogenetics of clozapine treatment response and side-effects in schizophrenia: an update". Expert Opinion on Drug Metabolism & Toxicology. 11 (11): 1709–31. doi:10.1517/17425255.2015.1075003. PMID 26364648. S2CID 207492339.
- Picchioni MM, Murray RM (July 2007). "Schizophrenia". BMJ. 335 (7610): 91–5. doi:10.1136/bmj.39227.616447.BE. PMC 1914490. PMID 17626963.
- Adam RL, Sidi H, Midin M, et al. (2018). "The Role of Atypical Antipsychotics in Sexuality: Road to Recovery in Schizophrenia". Current Drug Targets. 19 (12): 1402–1411. doi:10.2174/1389450118666170502130126. PMID 28464773. S2CID 41487184.
- Muench J, Hamer AM (March 2010). "Adverse effects of antipsychotic medications". American Family Physician. 81 (5): 617–22. PMID 20187598.
- Praharaj SK, Jana AK, Goyal N, Sinha VK (March 2011). "Metformin for olanzapine-induced weight gain: a systematic review and meta-analysis". British Journal of Clinical Pharmacology. 71 (3): 377–82. doi:10.1111/j.1365-2125.2010.03783.x. PMC 3045546. PMID 21284696.
- Yu O, Lu M, Lai T, Hahn M, Agarwal SM, O'Donoghue B, Ebdrup BH, Siskind D (2024). "Metformin co-commencement at time of antipsychotic initiation for attenuation of weight gain: a systematic review and meta-analysis". Therapeutic Advances in Psychopharmacology. 14: 20451253241255476. doi:10.1177/20451253241255476. PMC 11141220. PMID 38827016.
- Lieberman JA (2004). "Managing anticholinergic side effects". Primary Care Companion to the Journal of Clinical Psychiatry. 6 (Suppl 2): 20–3. PMC 487008. PMID 16001097.
- University of Hong Kong (6 September 2022). "Antipsychotic Use Associated With Elevated Risk of Breast Cancer".
- Koller EA, Doraiswamy PM (July 2002). "Olanzapine-associated diabetes mellitus". Pharmacotherapy. 22 (7): 841–52. doi:10.1592/phco.22.11.841.33629. PMID 12126218. S2CID 27314943.
- Weston-Green K, Huang XF, Deng C (February 2010). "Sensitivity of the female rat to olanzapine-induced weight gain—far from the clinic?". Schizophrenia Research. 116 (2–3): 299–300. doi:10.1016/j.schres.2009.09.034. PMID 19840894. S2CID 30384727.
- ^ Brunton LL, Chabner B, Knollmann BC, eds. (2011). Goodman & Gilman's The Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill. ISBN 978-0-07-162442-8.
- Weston-Green K, Huang XF, Deng C (2012). Chang AY (ed.). "Alterations to melanocortinergic, GABAergic and cannabinoid neurotransmission associated with olanzapine-induced weight gain". PLOS ONE. 7 (3): e33548. Bibcode:2012PLoSO...733548W. doi:10.1371/journal.pone.0033548. PMC 3306411. PMID 22438946.
- Koller EA, Cross JT, Doraiswamy PM, Malozowski SN (September 2003). "Pancreatitis associated with atypical antipsychotics: from the Food and Drug Administration's MedWatch surveillance system and published reports". Pharmacotherapy. 23 (9): 1123–30. doi:10.1592/phco.23.10.1123.32759. PMID 14524644. S2CID 39945446.
- Weinmann S, Read J, Aderhold V (August 2009). "Influence of antipsychotics on mortality in schizophrenia: systematic review". Schizophrenia Research. 113 (1): 1–11. doi:10.1016/j.schres.2009.05.018. PMID 19524406. S2CID 8143217.
- Joukamaa M, Heliövaara M, Knekt P, Aromaa A, Raitasalo R, Lehtinen V (February 2006). "Schizophrenia, neuroleptic medication and mortality". The British Journal of Psychiatry. 188 (2): 122–7. doi:10.1192/bjp.188.2.122. PMID 16449697.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel (April 2012). "American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults". Journal of the American Geriatrics Society. 60 (4): 616–31. doi:10.1111/j.1532-5415.2012.03923.x. PMC 3571677. PMID 22376048.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - Medford N, Sierra M, Baker D, David A (2005). "Understanding and treating depersonalisation disorder". Advances in Psychiatric Treatment. 11 (2): 92–100. doi:10.1192/apt.11.2.92. Archived from the original on 16 July 2011.
- Patrick V, Levin E, Schleifer S (July 2005). "Antipsychotic polypharmacy: is there evidence for its use?". Journal of Psychiatric Practice. 11 (4): 248–57. doi:10.1097/00131746-200507000-00005. PMID 16041235. S2CID 43114395.
- Ito H, Koyama A, Higuchi T (September 2005). "Polypharmacy and excessive dosing: psychiatrists' perceptions of antipsychotic drug prescription". The British Journal of Psychiatry. 187 (3): 243–7. doi:10.1192/bjp.187.3.243. PMID 16135861.
- Leung JC, Ng DW, Chu RY, Chan EW, Huang L, Lum DH, et al. (September 2022). "Association of antipsychotic use with breast cancer: a systematic review and meta-analysis of observational studies with over 2 million individuals". Epidemiology and Psychiatric Sciences. 31: e61. doi:10.1017/S2045796022000476. PMC 9483823. PMID 36059215.
- Vita A, De Peri L, Deste G, Sacchetti E (November 2012). "Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies". Translational Psychiatry. 2 (11): e190. doi:10.1038/tp.2012.116. PMC 3565772. PMID 23168990.
- Radua J, Borgwardt S, Crescini A, Mataix-Cols D, Meyer-Lindenberg A, McGuire PK, Fusar-Poli P (November 2012). "Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication". Neuroscience and Biobehavioral Reviews. 36 (10): 2325–33. doi:10.1016/j.neubiorev.2012.07.012. PMID 22910680.
- Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA (September 2005). "The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys". Neuropsychopharmacology. 30 (9): 1649–1661. doi:10.1038/sj.npp.1300710. PMID 15756305. S2CID 205679212.
- Parks J, Radke A, Parker G, Foti ME, Eilers R, Diamond M, et al. (September 2009). "Principles of antipsychotic prescribing for policy makers, circa 2008. Translating knowledge to promote individualized treatment". Schizophrenia Bulletin. 35 (5): 931–936. doi:10.1093/schbul/sbn019. PMC 2728806. PMID 18385207.
- Hirose S (2003). "The causes of underdiagnosing akathisia". Schizophrenia Bulletin. 29 (3): 547–58. CiteSeerX 10.1.1.618.3326. doi:10.1093/oxfordjournals.schbul.a007027. PMID 14609248.
- Rehse M, Bartolovic M, Baum K, Richter D, Weisbrod M, Roesch-Ely D (2016). "Influence of Antipsychotic and Anticholinergic Loads on Cognitive Functions in Patients with Schizophrenia". Schizophrenia Research and Treatment. 2016: 8213165. doi:10.1155/2016/8213165. PMC 4842070. PMID 27144021.
- Tampi RR, Tampi DJ, Balachandran S, Srinivasan S (September 2016). "Antipsychotic use in dementia: a systematic review of benefits and risks from meta-analyses". Therapeutic Advances in Chronic Disease. 7 (5): 229–245. doi:10.1177/2040622316658463. PMC 4994396. PMID 27583123.
- MacKenzie NE, Kowalchuk C, Agarwal SM, Costa-Dookhan KA, Caravaggio F, Gerretsen P, et al. (5 December 2018). "Antipsychotics, Metabolic Adverse Effects, and Cognitive Function in Schizophrenia". Frontiers in Psychiatry. 9: 622. doi:10.3389/fpsyt.2018.00622. PMC 6290646. PMID 30568606.
- Mueller C, John C, Perera G, Aarsland D, Ballard C, Stewart R (January 2021). "Antipsychotic use in dementia: the relationship between neuropsychiatric symptom profiles and adverse outcomes". European Journal of Epidemiology. 36 (1): 89–101. doi:10.1007/s10654-020-00643-2. PMC 7847435. PMID 32415541.
- Steinberg M, Lyketsos CG (September 2012). "Atypical antipsychotic use in patients with dementia: managing safety concerns". The American Journal of Psychiatry. 169 (9): 900–906. doi:10.1176/appi.ajp.2012.12030342. PMC 3516138. PMID 22952071.
- ^ Durrani US, Vasireddy S, Arshad MZ, Paracha A, Paracha MA, Waheed F, Abid A, Siddiqui Z, Thomure M (November 2023). "The Effect of Antipsychotics on Prolactinoma Growth: A Radiological and Serological Analysis". Cureus. 15 (11): e49342. doi:10.7759/cureus.49342. PMC 10748855. PMID 38143631.
- ^ Edinoff AN, Silverblatt NS, Vervaeke HE, Horton CC, Girma E, Kaye AD, Kaye A, Kaye JS, Garcia AJ, Neuchat EE, Eubanks TN, Varrassi G, Viswanath O, Urits I (March 2021). "Hyperprolactinemia, Clinical Considerations, and Infertility in Women on Antipsychotic Medications". Psychopharmacol Bull. 51 (2): 131–148. PMC 8146565. PMID 34092827.
- Joint Formulary Committee B, ed. (March 2009). "4.2.1". British National Formulary (57 ed.). United Kingdom: Royal Pharmaceutical Society of Great Britain. p. 192. ISBN 978-0-85369-845-6.
Withdrawal of antipsychotic drugs after long-term therapy should always be gradual and closely monitored to avoid the risk of acute withdrawal syndromes or rapid relapse.
- ^ Haddad P, Haddad PM, Dursun S, Deakin B (2004). Adverse Syndromes and Psychiatric Drugs: A Clinical Guide. OUP Oxford. pp. 207–216. ISBN 978-0-19-852748-0.
- ^ Moncrieff J (July 2006). "Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal-related relapse". Acta Psychiatrica Scandinavica. 114 (1): 3–13. doi:10.1111/j.1600-0447.2006.00787.x. PMID 16774655. S2CID 6267180.
- Sacchetti E, Vita A, Siracusano A, Fleischhacker W (2013). Adherence to Antipsychotics in Schizophrenia. Springer Science & Business Media. p. 85. ISBN 978-88-470-2679-7.
- Nakata Y, Kanahara N, Iyo M (December 2017). "Dopamine supersensitivity psychosis in schizophrenia: Concepts and implications in clinical practice". Journal of Psychopharmacology. 31 (12): 1511–1518. doi:10.1177/0269881117728428. PMID 28925317. S2CID 1957881.
- Glazer WM (2000). "Expected incidence of tardive dyskinesia associated with atypical antipsychotics". The Journal of Clinical Psychiatry. 61 (Suppl 4): 21–6. PMID 10739327.
- Lambert TJ (2007). "Switching antipsychotic therapy: what to expect and clinical strategies for improving therapeutic outcomes". The Journal of Clinical Psychiatry. 68 (Suppl 6): 10–3. PMID 17650054.
- BMJ Group (March 2009). "4.2.1". British National Formulary (57 ed.). United Kingdom: Royal Pharmaceutical Society of Great Britain. p. 192.
Withdrawal of antipsychotic drugs after long-term therapy should always be gradual and closely monitored to avoid the risk of acute withdrawal syndromes or rapid relapse.
- "Up to 100,000 on antipsychotics with no review". Health Service Journal. 27 January 2020. Retrieved 22 March 2020.
- Seeman P (January 2004). "Atypical Antipsychotics: Mechanism of Action". Focus. 2 (1): 48–58. doi:10.1176/foc.2.1.48.
- ^ Onrust SV, McClellan K (2001). "Perospirone". CNS Drugs. 15 (4): 329–37, discussion 338. doi:10.2165/00023210-200115040-00006. PMID 11463136. S2CID 262520276.
- Nemeroff CB, Lieberman JA, Weiden PJ, Harvey PD, Newcomer JW, Schatzberg AF, Kilts CD, Daniel DG (November 2005). "From clinical research to clinical practice: a 4-year review of ziprasidone". CNS Spectrums. 10 (11 Suppl 17): 1–20. doi:10.1017/S1092852900019842. PMID 16381088. S2CID 26738197.
- Winans E (1 December 2003). "Aripiprazole". American Journal of Health-System Pharmacy. 60 (23): 2437–2445. doi:10.1093/ajhp/60.23.2437. ISSN 1079-2082. PMID 14686220.
- "Caplyta (lumateperone) FDA Approval History". Drugs.com. Retrieved 20 November 2022.
- Swainston Harrison T, Perry CM (2004). "Aripiprazole: a review of its use in schizophrenia and schizoaffective disorder". Drugs. 64 (15): 1715–36. doi:10.2165/00003495-200464150-00010. PMID 15257633.
- Pickar D, Litman RE, Konicki PE, Wolkowitz OM, Breier A (1990). "Neurochemical and Neural Mechanisms of Positive and Negative Symptoms in Schizophrenia". Modern Problems of Pharmacopsychiatry. Modern Trends in Pharmacopsychiatry. 24: 124–151. doi:10.1159/000418015. ISBN 978-3-8055-5050-5. PMID 1970851.
- Liemburg EJ, Knegtering H, Klein HC, Kortekaas R, Aleman A (June 2012). "Antipsychotic medication and prefrontal cortex activation: a review of neuroimaging findings". European Neuropsychopharmacology. 22 (6): 387–400. doi:10.1016/j.euroneuro.2011.12.008. PMID 22300864. S2CID 24877454.
- ^ McDonald C, Murphy KC (March 2003). "The new genetics of schizophrenia". The Psychiatric Clinics of North America. 26 (1): 41–63. doi:10.1016/S0193-953X(02)00030-8. PMID 12683259.
- Schmidt CJ, Sorensen SM, Kehne JH, Carr AA, Palfreyman MG (1995). "The role of 5-HT2A receptors in antipsychotic activity". Life Sciences. 56 (25): 2209–22. doi:10.1016/0024-3205(95)00210-W. PMID 7791509.
- Tiwari P, Panik R, Bhattacharya A, Ahirwar D, Chandy A (December 2012). "Evidences of possible side effects of neuroleptic drugs: A systematic review". Asian Pacific Journal of Reproduction. 1 (4): 330–336. doi:10.1016/s2305-0500(13)60105-0. ISSN 2305-0500.
- ^ Stahl SM (2003). "Describing an Atypical Antipsychotic: Receptor Binding and Its Role in Pathophysiology" (PDF). Primary Care Companion to the Journal of Clinical Psychiatry. 5 (Suppl. 3): 9–13. Archived (PDF) from the original on 9 October 2022.
- Meltzer HY (2012). "Serotonergic Mechanisms as Targets for Existing and Novel Antipsychotics". Current Antipsychotics. Handbook of Experimental Pharmacology. Vol. 212. pp. 87–124. doi:10.1007/978-3-642-25761-2_4. ISBN 978-3-642-25760-5. PMID 23129329.
- Li M (August 2016). "Antipsychotic-induced sensitization and tolerance: Behavioral characteristics, developmental impacts, and neurobiological mechanisms". Journal of Psychopharmacology. 30 (8): 749–770. doi:10.1177/0269881116654697. PMC 4944179. PMID 27371498.
- Nawaratne V, McLaughlin SP, Mayer FP, Gichi Z, Mastriano A, Carvelli L (2021). "Prolonged Amphetamine Exposures Increase the Endogenous Human Dopamine Receptors 2 at the Cellular Membrane in Cells Lacking the Dopamine Transporter". Frontiers in Cellular Neuroscience. 15: 681539. doi:10.3389/fncel.2021.681539. PMC 8427050. PMID 34512264.
- Orejarena MJ, Lanfumey L, Maldonado R, Robledo P (August 2011). "Involvement of 5-HT2A receptors in MDMA reinforcement and cue-induced reinstatement of MDMA-seeking behaviour". The International Journal of Neuropsychopharmacology. 14 (7): 927–940. doi:10.1017/S1461145710001215. hdl:10230/25548. PMID 20942998.
- ^ Sanchez M (May 2012). Farmacología y endocrinología del comportamiento. Editorial UOC. pp. 148–149. ISBN 978-84-9788-424-2. Archived from the original on 25 November 2017.
- Fischer J, Ganellin CR (13 December 2006). Analogue-based Drug Discovery. John Wiley & Sons. pp. 305–. ISBN 978-3-527-60749-5.
- ^ Lidow MS (22 June 2000). Neurotransmitter Receptors in Actions of Antipsychotic Medications. CRC Press. pp. 23–. ISBN 978-1-4200-4177-4.
- ^ Silvestre JS, Prous J (June 2005). "Research on adverse drug events. I. Muscarinic M3 receptor binding affinity could predict the risk of antipsychotics to induce type 2 diabetes". Methods and Findings in Experimental and Clinical Pharmacology. 27 (5): 289–304. doi:10.1358/mf.2005.27.5.908643. PMID 16082416.
- "Aripiprazole lauroxil – Alkermes". AdisInsight. Springer Nature Switzerland AG.
- "Asenapine". AdisInsight. Springer Nature Switzerland AG.
- "Blonanserin – Sumitomo Dainippon Pharma". AdisInsight. Springer Nature Switzerland AG.
- "Brexpiprazole – Lundbeck/Otsuka". AdisInsight. Springer Nature Switzerland AG. Archived from the original on 11 October 2016. Retrieved 27 September 2017.
- "Cariprazine – Gedeon Richter". AdisInsight. Springer Nature Switzerland AG. Archived from the original on 18 August 2017. Retrieved 7 May 2017.
- ^ Csernansky JG (6 December 2012). Antipsychotics. Springer Science & Business Media. pp. 360–. ISBN 978-3-642-61007-3.
- William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. pp. 1077–. ISBN 978-0-8155-1856-3.
- ^ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. pp. 1102–. ISBN 978-0-8155-1856-3.
- Protiva M (2010). "ChemInform Abstract: Fifty Years in Chemical Drug Research". ChemInform. 23 (9): no. doi:10.1002/chin.199209338. ISSN 0931-7597.
- Melich H (April 1971). "". Časopis lékařů českých (in Czech). 110 (17): 404–5. PMID 5576292.
- ^ Drugs Available Abroad. Gale Research. 1991. p. 52,169. ISBN 978-0-8103-7177-4. Archived from the original on 22 July 2017.
- Geller V, Gorzaltsan I, Shleifer T, Belmaker RH, Bersudsky Y (December 2005). "Clotiapine compared with chlorpromazine in chronic schizophrenia". Schizophrenia Research. 80 (2–3): 343–7. doi:10.1016/j.schres.2005.07.007. PMID 16126373. S2CID 22340010.
- Bourin M, Dailly E, Hascöet M (2004). "Preclinical and clinical pharmacology of cyamemazine: anxiolytic effects and prevention of alcohol and benzodiazepine withdrawal syndrome". CNS Drug Reviews. 10 (3): 219–29. doi:10.1111/j.1527-3458.2004.tb00023.x. PMC 6741725. PMID 15492772.
- Lamberth C, Dinges J (16 August 2012). Bioactive Heterocyclic Compound Classes: Pharmaceuticals. John Wiley & Sons. pp. 3–. ISBN 978-3-527-66447-4.
- ^ Pedersen V (1996). "Thioxanthene antipsychotics". European Psychiatry. 11: 236s. doi:10.1016/0924-9338(96)88706-2. ISSN 0924-9338. S2CID 247414112.
- "Iloperidone – Vanda Pharmaceuticals". AdisInsight. Springer Nature Switzerland AG. Archived from the original on 11 December 2015. Retrieved 27 September 2017.
- ^ Shorter E (2009). Before Prozac: The Troubled History of Mood Disorders in Psychiatry. Oxford University Press, USA. pp. 14–. ISBN 978-0-19-536874-1.
- Glazer WM (1999). "Does loxapine have "atypical" properties? Clinical evidence". The Journal of Clinical Psychiatry. 60 (Suppl 10): 42–6. PMID 10340686.
- "Lurasidone – Sumitomo Dainippon Pharma". AdisInsight. Springer Nature Switzerland AG. Archived from the original on 10 May 2016. Retrieved 7 May 2017.
- "Nemonapride". AdisInsight. Springer Nature Switzerland AG.
- "Paliperidone – Johnson & Johnson". AdisInsight. Springer Nature Switzerland AG.
- "Paliperidone palmitate – Johnson & Johnson". AdisInsight. Springer Nature Switzerland AG. Archived from the original on 30 October 2016. Retrieved 27 September 2017.
- ^ Vardanyan R (12 June 2017). Piperidine-Based Drug Discovery. Elsevier Science. pp. 158, 195–. ISBN 978-0-12-813428-3.
- Roelofs GA (1974). "Penfluridol (R 16341) as a maintenance therapy in chronic psychotic patients: a double-blind clinical evaluation". Acta Psychiatrica Scandinavica. 50 (2): 219–24. doi:10.1111/j.1600-0447.1974.tb08210.x. PMID 4604881. S2CID 40524276.
- Leucht S, Helfer B, Hartung B (January 2014). "Perazine for schizophrenia". The Cochrane Database of Systematic Reviews (1): CD002832. doi:10.1002/14651858.CD002832.pub3. PMC 11015532. PMID 24425538.
- Matar HE, Almerie MQ, Makhoul S, Xia J, Humphreys P (May 2014). "Pericyazine for schizophrenia". The Cochrane Database of Systematic Reviews (5): CD007479. doi:10.1002/14651858.CD007479.pub2. PMC 11023599. PMID 24825770.
- "Perospirone". AdisInsight. Springer Nature Switzerland AG.
- "Pimavanserin – ACADIA Pharmaceuticals". AdisInsight. Springer Nature Switzerland AG. Archived from the original on 25 September 2017. Retrieved 27 September 2017.
- William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. pp. 2935–. ISBN 978-0-8155-1856-3.
- William Andrew Publishing (2007). Pharmaceutical Manufacturing Encyclopedia. William Andrew Pub. p. 3059. ISBN 978-0-8155-1526-5.
- William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. pp. 3129–. ISBN 978-0-8155-1856-3.
- William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. pp. 3214–. ISBN 978-0-8155-1856-3.
- Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- Joint Formulary Committee (2013). British National Formulary (BNF) (65 ed.). London, UK: Pharmaceutical Press. ISBN 978-0-85711-084-8.
- Isbister GK, Balit CR, Macleod D, Duffull SB (August 2010). "Amisulpride overdose is frequently associated with QT prolongation and torsades de pointes". Journal of Clinical Psychopharmacology. 30 (4): 391–5. doi:10.1097/JCP.0b013e3181e5c14c. PMID 20531221. S2CID 205710487.
- ^ Deeks ED, Keating GM (January 2010). "Blonanserin: a review of its use in the management of schizophrenia". CNS Drugs. 24 (1): 65–84. doi:10.2165/11202620-000000000-00000. PMID 20030420. S2CID 23464075.
- ^ Tenjin T, Miyamoto S, Ninomiya Y, Kitajima R, Ogino S, Miyake N, Yamaguchi N (2013). "Profile of blonanserin for the treatment of schizophrenia". Neuropsychiatric Disease and Treatment. 9: 587–94. doi:10.2147/NDT.S34433. PMC 3677929. PMID 23766647.
- "Clozapine". Martindale: The Complete Drug Reference. Royal Pharmaceutical Society of Great Britain. 30 January 2013. Retrieved 2 November 2013.
- Matar HE, Almerie MQ, Sampson S (June 2018). "Fluphenazine (oral) versus placebo for schizophrenia". The Cochrane Database of Systematic Reviews. 2020 (7): CD006352. doi:10.1002/14651858.CD006352.pub3. PMC 3796096. PMID 29893410.
- Chakrabarti A, Bagnall A, Chue P, Fenton M, Palaniswamy V, Wong W, Xia J (October 2007). Chakrabarti A (ed.). "Loxapine for schizophrenia". The Cochrane Database of Systematic Reviews. 2007 (4): CD001943. doi:10.1002/14651858.CD001943.pub2. PMC 7017975. PMID 17943763.
- Harvey PD, Ogasa M, Cucchiaro J, Loebel A, Keefe RS (April 2011). "Performance and interview-based assessments of cognitive change in a randomized, double-blind comparison of lurasidone vs. ziprasidone". Schizophrenia Research. 127 (1–3): 188–94. doi:10.1016/j.schres.2011.01.004. PMID 21277745. S2CID 8805912.
- Röhricht F, Gadhia S, Alam R, Willis M (2012). "Auditing clinical outcomes after introducing off-licence prescribing of atypical antipsychotic melperone for patients with treatment refractory schizophrenia". TheScientificWorldJournal. 2012: 1–5. doi:10.1100/2012/512047. PMC 3330679. PMID 22566771.
- ^ "Molindone Hydrochloride". Martindale: The Complete Drug Reference. The Royal Pharmaceutical Society of Great Britain. 30 January 2013. Retrieved 5 November 2013.
- Leucht S, Helfer B, Hartung B (January 2014). "Perazine for schizophrenia". The Cochrane Database of Systematic Reviews (1): CD002832. doi:10.1002/14651858.CD002832.pub3. PMC 11015532. PMID 24425538.
- Onrust SV, McClellan K (April 2001). "Perospirone". CNS Drugs. 15 (4): 329–37, discussion 338. doi:10.2165/00023210-200115040-00006. PMID 11463136. S2CID 262520276.
- ^ Brayfield A, ed. (23 September 2011). "Perospirone". Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. Retrieved 3 November 2013.
- Hartung B, Sampson S, Leucht S (March 2015). "Perphenazine for schizophrenia". The Cochrane Database of Systematic Reviews. 2015 (3): CD003443. doi:10.1002/14651858.CD003443.pub3. PMC 7173727. PMID 25749632.
- Mothi M, Sampson S (November 2013). "Pimozide for schizophrenia or related psychoses". The Cochrane Database of Systematic Reviews (11): CD001949. doi:10.1002/14651858.CD001949.pub3. PMID 24194433.
- Wang J, Sampson S (April 2014). "Sulpiride versus placebo for schizophrenia". The Cochrane Database of Systematic Reviews (4): CD007811. doi:10.1002/14651858.CD007811.pub2. PMC 11195628. PMID 24729184.
- Fenton M, Rathbone J, Reilly J, Sultana A (July 2007). Reilly J (ed.). "Thioridazine for schizophrenia". The Cochrane Database of Systematic Reviews. 2007 (3): CD001944. doi:10.1002/14651858.CD001944.pub2. PMC 6718212. PMID 17636691.
- Fornaro P, Calabria G, Corallo G, Picotti GB (July 2002). "Pathogenesis of degenerative retinopathies induced by thioridazine and other antipsychotics: a dopamine hypothesis". Documenta Ophthalmologica. Advances in Ophthalmology. 105 (1): 41–9. doi:10.1023/A:1015768114192. PMID 12152801. S2CID 23618581.
- Marques LO, Lima MS, Soares BG (2004). de Oliveira Marques L (ed.). "Trifluoperazine for schizophrenia". The Cochrane Database of Systematic Reviews. 2004 (1): CD003545. doi:10.1002/14651858.CD003545.pub2. PMC 7003674. PMID 14974020.
- "Zotepine". Martindale: The Complete Drug Reference. Royal Pharmaceutical Society of Great Britain. 16 August 2013. Retrieved 2 November 2013.
- Cipriani A, Barbui C, Salanti G, Rendell J, Brown R, Stockton S, et al. (October 2011). "Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis". Lancet. 378 (9799): 1306–1315. doi:10.1016/S0140-6736(11)60873-8. PMID 21851976. S2CID 25512763.
- Citrome L (2013). "Addressing the need for rapid treatment of agitation in schizophrenia and bipolar disorder: focus on inhaled loxapine as an alternative to injectable agents". Therapeutics and Clinical Risk Management. 9: 235–245. doi:10.2147/TCRM.S31484. PMC 3665578. PMID 23723707.
- Cruz N, Sanchez-Moreno J, Torres F, Goikolea JM, Valentí M, Vieta E (February 2010). "Efficacy of modern antipsychotics in placebo-controlled trials in bipolar depression: a meta-analysis". The International Journal of Neuropsychopharmacology. 13 (1): 5–14. doi:10.1017/S1461145709990344. hdl:2445/53243. PMID 19638254.
- Popovic D, Reinares M, Goikolea JM, Bonnin CM, Gonzalez-Pinto A, Vieta E (May 2012). "Polarity index of pharmacological agents used for maintenance treatment of bipolar disorder". European Neuropsychopharmacology. 22 (5): 339–346. doi:10.1016/j.euroneuro.2011.09.008. PMID 22000157. S2CID 8067809.
- Vieta E, Günther O, Locklear J, Ekman M, Miltenburger C, Chatterton ML, et al. (September 2011). "Effectiveness of psychotropic medications in the maintenance phase of bipolar disorder: a meta-analysis of randomized controlled trials". The International Journal of Neuropsychopharmacology. 14 (8): 1029–1049. doi:10.1017/S1461145711000885. hdl:2445/51726. PMID 21733231.
- Komossa K, Depping AM, Gaudchau A, Kissling W, Leucht S (December 2010). "Second-generation antipsychotics for major depressive disorder and dysthymia". The Cochrane Database of Systematic Reviews (12): CD008121. doi:10.1002/14651858.CD008121.pub2. PMID 21154393.
- Arafat SM, Rahman SM, Haque MM, Shah MA, Algin S, Nahar JS (2016). "Clozapine Can Be the Good Option in Resistant Mania". Case Reports in Psychiatry. 2016: 3081704. doi:10.1155/2016/3081704. PMC 4976188. PMID 27525148.
- Wilkowska A, Cubała WJ (9 October 2019). "Clozapine As Transformative Treatment In Bipolar Patients". Neuropsychiatric Disease and Treatment. 15: 2901–2905. doi:10.2147/NDT.S227196. PMC 6790347. PMID 31632038.
- https://www.has-sante.fr/upload/docs/application/pdf/2012-04/anti_psychotiques_rapport.pdf
- Kishi T, Iwata N (September 2013). "Efficacy and tolerability of perospirone in schizophrenia: a systematic review and meta-analysis of randomized controlled trials". CNS Drugs. 27 (9): 731–41. doi:10.1007/s40263-013-0085-7. PMID 23812802. S2CID 11543666.
- Roth BL, Driscol J (12 January 2011). "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Archived from the original on 8 November 2013. Retrieved 11 November 2013.
- Shahid M, Walker GB, Zorn SH, Wong EH (January 2009). "Asenapine: a novel psychopharmacologic agent with a unique human receptor signature". Journal of Psychopharmacology. 23 (1): 65–73. doi:10.1177/0269881107082944. PMID 18308814. S2CID 206489515.
- ^ Ishiyama T, Tokuda K, Ishibashi T, Ito A, Toma S, Ohno Y (October 2007). "Lurasidone (SM-13496), a novel atypical antipsychotic drug, reverses MK-801-induced impairment of learning and memory in the rat passive-avoidance test". European Journal of Pharmacology. 572 (2–3): 160–70. doi:10.1016/j.ejphar.2007.06.058. PMID 17662268.
- Ishibashi T, Horisawa T, Tokuda K, Ishiyama T, Ogasa M, Tagashira R, Matsumoto K, Nishikawa H, Ueda Y, Toma S, Oki H, Tanno N, Saji I, Ito A, Ohno Y, Nakamura M (July 2010). "Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity". The Journal of Pharmacology and Experimental Therapeutics. 334 (1): 171–81. doi:10.1124/jpet.110.167346. PMID 20404009. S2CID 12893717.
- López-Muñoz F, Alamo C (September 2013). "Active metabolites as antidepressant drugs: the role of norquetiapine in the mechanism of action of quetiapine in the treatment of mood disorders". Frontiers in Psychiatry. 4: 102. doi:10.3389/fpsyt.2013.00102. PMC 3770982. PMID 24062697.
- "Medscape home page". Medscape. WebMD. Archived from the original on 13 November 2013.
- "Therapeutic Goods Administration home page". Therapeutic Goods Administration. Department of Health (Australia). Archived from the original on 21 April 2013.
- "Daily Med home page". Daily Med. United States National Library of Medicine. Archived from the original on 18 June 2013.
- "electronic Medicines Compendium (eMC) home page". electronic Medicines Compendium. Datapharm. Archived from the original on 27 November 2013.
- Wen YG, Shang DW, Xie HZ, Wang XP, Ni XJ, Zhang M, Lu W, Qiu C, Liu X, Li FF, Li X, Luo FT (March 2013). "Population pharmacokinetics of blonanserin in Chinese healthy volunteers and the effect of the food intake". Human Psychopharmacology. 28 (2): 134–41. doi:10.1002/hup.2290. PMID 23417765. S2CID 12623938.
- Borgström L, Larsson H, Molander L (1982). "Pharmacokinetics of parenteral and oral melperone in man". European Journal of Clinical Pharmacology. 23 (2): 173–6. doi:10.1007/BF00545974. PMID 7140807. S2CID 36697288.
- Callaghan JT, Bergstrom RF, Ptak LR, Beasley CM (September 1999). "Olanzapine. Pharmacokinetic and pharmacodynamic profile". Clinical Pharmacokinetics. 37 (3): 177–93. doi:10.2165/00003088-199937030-00001. PMID 10511917.
- Vermeir M, Naessens I, Remmerie B, Mannens G, Hendrickx J, Sterkens P, Talluri K, Boom S, Eerdekens M, van Osselaer N, Cleton A (April 2008). "Absorption, metabolism, and excretion of paliperidone, a new monoaminergic antagonist, in humans". Drug Metabolism and Disposition. 36 (4): 769–79. doi:10.1124/dmd.107.018275. PMID 18227146. S2CID 41656.
- DeVane CL, Nemeroff CB (2001). "Clinical pharmacokinetics of quetiapine: an atypical antipsychotic". Clinical Pharmacokinetics. 40 (7): 509–22. doi:10.2165/00003088-200140070-00003. PMID 11510628. S2CID 33802262.
- Wiesel FA, Alfredsson G, Ehrnebo M, Sedvall G (May 1980). "The pharmacokinetics of intravenous and oral sulpiride in healthy human subjects". European Journal of Clinical Pharmacology. 17 (5): 385–91. doi:10.1007/BF00558453. PMID 7418717. S2CID 28141135.
- Prakash A, Lamb HM (January 1998). "Zotepine: A Review of its Pharmacodynamic and Pharmacokinetic Properties and Therapeutic Efficacy in the Management of Schizophrenia". CNS Drugs. 9 (2): 153–175. doi:10.2165/00023210-199809020-00006.
- Product Information: Nipolept(R), zotepine. Klinge Pharma GmbH, Munich, 1996.
- Parent M, Toussaint C, Gilson H (1983). "Long-term treatment of chronic psychotics with bromperidol decanoate: clinical and pharmacokinetic evaluation". Current Therapeutic Research. 34 (1): 1–6.
- ^ Jørgensen A, Overø KF (1980). "Clopenthixol and flupenthixol depot preparations in outpatient schizophrenics. III. Serum levels". Acta Psychiatrica Scandinavica. Supplementum. 279: 41–54. doi:10.1111/j.1600-0447.1980.tb07082.x. PMID 6931472.
- ^ Reynolds JE (1993). "Anxiolytic sedatives, hypnotics and neuroleptics.". Martindale: The Extra Pharmacopoeia (30th ed.). London: Pharmaceutical Press. pp. 364–623.
- Ereshefsky L, Saklad SR, Jann MW, Davis CM, Richards A, Seidel DR (May 1984). "Future of depot neuroleptic therapy: pharmacokinetic and pharmacodynamic approaches". The Journal of Clinical Psychiatry. 45 (5 Pt 2): 50–9. PMID 6143748.
- ^ Curry SH, Whelpton R, de Schepper PJ, Vranckx S, Schiff AA (April 1979). "Kinetics of fluphenazine after fluphenazine dihydrochloride, enanthate and decanoate administration to man". British Journal of Clinical Pharmacology. 7 (4): 325–31. doi:10.1111/j.1365-2125.1979.tb00941.x. PMC 1429660. PMID 444352.
- Young D, Ereshefsky L, Saklad SR, Jann MW, Garcia N (1984). Explaining the pharmacokinetics of fluphenazine through computer simulations. (Abstract.). 19th Annual Midyear Clinical Meeting of the American Society of Hospital Pharmacists. Dallas, Texas.
- Janssen PA, Niemegeers CJ, Schellekens KH, Lenaerts FM, Verbruggen FJ, van Nueten JM, Marsboom RH, Hérin VV, Schaper WK (November 1970). "The pharmacology of fluspirilene (R 6218), a potent, long-acting and injectable neuroleptic drug". Arzneimittel-Forschung. 20 (11): 1689–98. PMID 4992598.
- Beresford R, Ward A (January 1987). "Haloperidol decanoate. A preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in psychosis". Drugs. 33 (1): 31–49. doi:10.2165/00003495-198733010-00002. PMID 3545764.
- Reyntigens AJ, Heykants JJ, Woestenborghs RJ, Gelders YG, Aerts TJ (1982). "Pharmacokinetics of haloperidol decanoate. A 2-year follow-up". International Pharmacopsychiatry. 17 (4): 238–46. doi:10.1159/000468580. PMID 7185768.
- Larsson M, Axelsson R, Forsman A (1984). "On the pharmacokinetics of perphenazine: a clinical study of perphenazine enanthate and decanoate". Current Therapeutic Research. 36 (6): 1071–88.
- The text reads: "When the patient lashes out against 'them' – THORAZINE (brand of chlorpromazine) quickly puts an end to his violent outburst. 'Thorazine' is especially effective when the psychotic episode is triggered by delusions or hallucinations. At the outset of treatment, Thorazine's combination of antipsychotic and sedative effects provides both emotional and physical calming. Assaultive or destructive behavior is rapidly controlled. As therapy continues, the initial sedative effect gradually disappears. But the antipsychotic effect continues, helping to dispel or modify delusions, hallucinations and confusion, while keeping the patient calm and approachable. SMITH KLINE AND FRENCH LABORATORIES leaders in psychopharmaceutical research."
- ^ Pieters T, Majerus B (December 2011). "The introduction of chlorpromazine in Belgium and the Netherlands (1951–1968); tango between old and new treatment features". Studies in History and Philosophy of Biological and Biomedical Sciences. 42 (4): 443–452. doi:10.1016/j.shpsc.2011.05.003. PMID 22035718. Archived from the original on 9 July 2017. Retrieved 26 February 2017.
- Healy D (2005). Psychiatric Drugs Explained (4th ed.). Britain: Elsevier Limited. pp. 8, 17.
- "tranquillizer, n". Oxford English Dictionary. 1989. Retrieved 9 August 2011.
- Healy D (2008). "The Intersection of Psychopharmacology and Psychiatry in the Second Half of the Twentieth Century". In Wallace ER, Gach J (eds.). History of Psychiatry and Medical Psychology. Boston, MA: Springer US. p. 421. doi:10.1007/978-0-387-34708-0_13. ISBN 978-0-387-34707-3.
- Owens DG (13 April 1999). A guide to the extrapyramidal side-effects of antipsychotic drugs. Cambridge University Press. p. 12. ISBN 978-0-521-63353-6. Archived from the original on 18 March 2016.
- Wilson S, Anderson K, Baldwin D, Dijk DJ, Espie A, Espie C, et al. (August 2019). "British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: An update". Journal of Psychopharmacology. 33 (8): 923–947. doi:10.1177/0269881119855343. PMID 31271339. S2CID 195797603.
- Tasman A (1997). Psychiatry Volume 2. Saunders. p. 956. ISBN 978-0-7216-5257-3.
- Ayd FJ (2000). "neuroleptic". Lexicon of Psychiatry, Neurology, and the Neurosciences. Lippincott Williams & Wilkins. p. 675. ISBN 978-0-7817-2468-5.
- R. Elliott Ingersoll, Carl F. Rak (2015): Psychopharmacology for Mental Health Professionals: An Integrative Approach, Cengage Learning, Boston, pp. 150-186 and 342-349, ISBN 9781305537231.
- Patel SC, Jakopac KA (2011). Manual of Psychiatric Nursing Skills. Jones & Bartlett Publishers. p. 317. ISBN 978-1-4496-1356-3.
- Drug Policy and the Public Good. OUP Oxford. 2010. p. 329. ISBN 978-0-19-955712-7.
- ^ Pipelineantipsychotic drugs to drive next market evolution (2009). Healthcarefinancenews.com (7 August 2009).
- Aparasu RR, Bhatara V (December 2006). "Antipsychotic use and expenditure in the United States". Psychiatric Services. 57 (12): 1693. doi:10.1176/appi.ps.57.12.1693. PMID 17158480.
- 2008 U.S. Sales and Prescription Information: Top Therapeutic Classes by U.S. Sales (PDF) Archived 16 April 2010 at the Wayback Machine. Imshealth.com.
- "Data show 'spiralling' antipsychotic price increases of up to 1,200% in past five years". Pharmaceutical Journal. 24 August 2022. Retrieved 29 September 2022.
- Gustafsson M, Karlsson S, Lövheim H (February 2013). "Inappropriate long-term use of antipsychotic drugs is common among people with dementia living in specialized care units". BMC Pharmacology & Toxicology. 14 (1): 10. doi:10.1186/2050-6511-14-10. PMC 3575309. PMID 23391323.
- Risky Antipsychotic Drugs Still Overprescribed In Nursing Homes, https://www.npr.org/sections/health-shots/2018/02/05/583435517/risky-antipsychotic-drugs-still-overprescribed-in-nursing-homes
- Atypical antipsychotics: overrated and overprescribed, Glen Spielsman, https://pharmaceutical-journal.com/article/opinion/atypical-antipsychotics-overrated-and-overprescribed
- Carrithers B, El-Mallakh RS (18 March 2020). "Transdermal Asenapine in Schizophrenia: A Systematic Review". Patient Preference and Adherence. 14: 1541–1551. doi:10.2147/PPA.S235104. PMC 7468370. PMID 32943849.
- ^ Bogart G (2011). "Abuse of second-generation antipsychotics: What prescribers need to know". Current Psychiatry. 10 (5): 77–79.
- James A (2 March 2008). "Myth of the antipsychotic". The Guardian.
- "GPs under 'pressure' to issue neuroleptics, claims professor". Chemist+Druggist. Archived from the original on 20 October 2020. Retrieved 1 May 2020.
- Triggle N (12 November 2009). "Dementia drug use 'killing many'".
- "UK study warns against anti-psychotics for dementia". Reuters. 12 November 2009.
- Hilzenrath DS (16 January 2010). "Justice suit accuses Johnson & Johnson of paying kickbacks". The Washington Post.
- ^ Wilson D (2 October 2010). "Side Effects May Include Lawsuits". The New York Times. Archived from the original on 5 October 2010.
- Wilson D (27 February 2009). "Drug Maker's E-Mail Released in Seroquel Lawsuit". The New York Times.
- Gosden R, Beder S (1 January 2001). "Pharmaceutical industry agenda setting in mental health policies". Ethical Human Sciences and Services. 3 (3): 147–159. doi:10.1891/1523-150X.3.3.147 (inactive 1 November 2024). PMID 15278977.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ American Psychiatric Association (September 2013). "Five Things Physicians and Patients Should Question". Choosing Wisely: an initiative of the ABIM Foundation. American Psychiatric Association. Archived from the original on 3 December 2013. Retrieved 30 December 2013., which cites
- American Psychiatric Association (2006). "Practice Guideline for the Treatment of Patients with Alzheimer's Disease and Other Dementias". APA Practice Guidelines for the Treatment of Psychiatric Disorders: Comprehensive Guidelines and Guideline Watches. Vol. 1 (Second ed.). doi:10.1176/appi.books.9780890423967.152139 (inactive 4 December 2024). ISBN 978-0-89042-336-3.
{{cite book}}: CS1 maint: DOI inactive as of December 2024 (link) - Aparasu RR, Bhatara V (December 2006). "Antipsychotic use and expenditure in the United States". Psychiatric Services. 57 (12): 1693. doi:10.1176/appi.ps.57.12.1693. PMID 17158480.
- Gitlin LN, Kales HC, Lyketsos CG (November 2012). "Nonpharmacologic management of behavioral symptoms in dementia". JAMA. 308 (19): 2020–9. doi:10.1001/jama.2012.36918. PMC 3711645. PMID 23168825.
- Maglione M, Maher AR, Hu J, Wang Z, Shanman R, Shekelle PG, Roth B, Hilton L, Suttorp MJ, Ewing BA, Motala A, Perry T (September 2011). "Off-Label Use of Atypical Antipsychotics: An Update". AHRQ Comparative Effectiveness Reviews. PMID 22132426.
- Richter T, Meyer G, Möhler R, Köpke S (December 2012). "Psychosocial interventions for reducing antipsychotic medication in care home residents". The Cochrane Database of Systematic Reviews. 12 (12): CD008634. doi:10.1002/14651858.cd008634.pub2. PMC 6492452. PMID 23235663. S2CID 42099598.
- American Psychiatric Association (2006). "Practice Guideline for the Treatment of Patients with Alzheimer's Disease and Other Dementias". APA Practice Guidelines for the Treatment of Psychiatric Disorders: Comprehensive Guidelines and Guideline Watches. Vol. 1 (Second ed.). doi:10.1176/appi.books.9780890423967.152139 (inactive 4 December 2024). ISBN 978-0-89042-336-3.
- American Geriatrics Society. "Ten Things Physicians and Patients Should Question". Choosing Wisely: an initiative of the ABIM Foundation. American Geriatrics Society. Archived from the original on 1 September 2013. Retrieved 1 August 2013., which cites
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel (April 2012). "American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults". Journal of the American Geriatrics Society. 60 (4): 616–31. doi:10.1111/j.1532-5415.2012.03923.x. PMC 3571677. PMID 22376048.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - NICE (8 May 2013). Dementia: Supporting people with dementia and their carers in health and social care Clinical guidelines, CG42. NICE. Archived from the original on 27 June 2013. Retrieved 23 October 2013.
- Maher AR, Maglione M, Bagley S, Suttorp M, Hu JH, Ewing B, Wang Z, Timmer M, Sultzer D, Shekelle PG (September 2011). "Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysis". JAMA. 306 (12): 1359–69. doi:10.1001/jama.2011.1360. PMID 21954480.
- Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, Lebowitz BD, Lyketsos CG, Ryan JM, Stroup TS, Sultzer DL, Weintraub D, Lieberman JA (October 2006). "Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease". The New England Journal of Medicine. 355 (15): 1525–38. doi:10.1056/nejmoa061240. PMID 17035647. S2CID 5861676.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel (April 2012). "American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults". Journal of the American Geriatrics Society. 60 (4): 616–31. doi:10.1111/j.1532-5415.2012.03923.x. PMC 3571677. PMID 22376048.
Further reading
- Fallon P, Dursun S, Deakin B (February 2012). "Drug-induced supersensitivity psychosis revisited: characteristics of relapse in treatment-compliant patients". Therapeutic Advances in Psychopharmacology. 2 (1): 13–22. doi:10.1177/2045125311431105. PMC 3736929. PMID 23983951.
External links
- Recommendations for the use of antipsychotics for treating psychosis, World Health Organization 2012
- Are atypical antipsychotics advantageous? – the case for, Australian Prescriber 2005 (note: pharmaceutical company conflict of interest statement at the end)
- Are atypical antipsychotics advantageous? – the case against, Australian Prescriber 2005
- First Generation Antipsychotics: An Introduction, Psychopharmacology Institute, 2012
- FDA Public Health Advisory – Public Health Advisory for Antipsychotic Drugs used for Treatment of Behavioral Disorders in Elderly Patients, fda.gov
- Antipsychotic Medication – information from mental health charity The Royal College of Psychiatrists
- (in Portuguese) FROTA LH. Fifty Years of Antipsychotic Drugs in Psychiatry. "Cinqüenta Anos de Medicamentos Antipsicóticos em Psiquiatria." 1st ed; Ebook: CD-Rom/On-Line Portuguese, ISBN 85-903827-1-0, File .pdf (Adobe Acrobat) 6Mb, Informática, Rio de Janeiro, August 2003, 486pp., medicina.ufrj.br
| Major chemical drug groups – based upon the Anatomical Therapeutic Chemical Classification System | |
|---|---|
| gastrointestinal tract / metabolism (A) | |
| blood and blood forming organs (B) | |
| cardiovascular system (C) | |
| skin (D) | |
| genitourinary system (G) | |
| endocrine system (H) | |
| infections and infestations (J, P, QI) | |
| malignant disease (L01–L02) | |
| immune disease (L03–L04) | |
| muscles, bones, and joints (M) | |
| brain and nervous system (N) |
|
| respiratory system (R) | |
| sensory organs (S) | |
| other ATC (V) | |
| Mood disorder | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Spectrum |
| ||||||||
| Symptoms | |||||||||
| Diagnosis | |||||||||
| Treatment |
| ||||||||
| History | |||||||||