| |
| Names | |
|---|---|
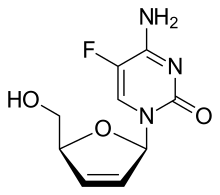
| IUPAC name 2′,3′-Didehydro-2′,3′-dideoxy-5-fluorocytidine | |
| Systematic IUPAC name 4-Amino-5-fluoro-1-pyrimidin-2(1H)-one | |
| Other names Reverset | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C9H10FN3O3 |
| Molar mass | 227.195 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Dexelvucitabine is a failed experimental agent for the management of human immunodeficiency virus infection. It is a cytidine nucleoside analog and nucleoside reverse transcriptase inhibitor. that inhibits HIV-1 replication in vitro. During phase II clinical trials there was some indication of a decreased mean viral load in patients with infected human immunodeficiency virus.
On April 3, 2006, Pharmasset and Incyte, the pharmaceutical companies developing dexelvucitabine, announced the decision to cease further trials and development of the drug due to an increased incidence of grade 4 hyperlipasemia (an excess of the pancreatic enzyme lipase in the bloodstream) in a phase II trial.
References
- ^ PubChem. "Dexelvucitabine". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-04-07.
- Hernandez-Santiago, Brenda I.; Mathew, Judy S.; Rapp, Kim L.; Grier, Jason P.; Schinazi, Raymond F. (June 2007). "Antiviral and Cellular Metabolism Interactions between Dexelvucitabine and Lamivudine". Antimicrobial Agents and Chemotherapy. 51 (6): 2130–2135. doi:10.1128/aac.01543-06. ISSN 0066-4804. PMC 1891415. PMID 17403996.
- Sobieszczyk, Magdalena E; Talley, Angela K; Wilkin, Timothy; Hammer, Scott M (2005-03-01). "Advances in antiretroviral therapy". Topics in HIV Medicine. 13 (1): 24–44. ISSN 2161-5845. PMID 15849370.
- Ryder, Neil S (2007-12-01). "Discontinued drugs in 2006: anti-infectives". Expert Opinion on Investigational Drugs. 16 (12): 1867–1878. doi:10.1517/13543784.16.12.1867. ISSN 1354-3784. PMID 18041997. S2CID 40129603.
This antiinfective drug article is a stub. You can help Misplaced Pages by expanding it. |