| |||
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name Difluorophosphate | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
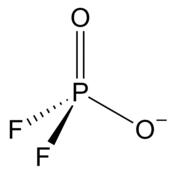
| Chemical formula | PO2F−2 | ||
| Molar mass | 100.97 g mol | ||
| Structure | |||
| Coordination geometry | Tetracoordinated at phosphorus atom | ||
| Molecular shape | Tetrahedral at phosphorus atom | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Difluorophosphate or difluorodioxophosphate or phosphorodifluoridate is an anion with formula PO2F−2. It has a single negative charge and resembles perchlorate (ClO−4) and monofluorosulfonate (SO3F) in shape and compounds. These ions are isoelectronic, along with tetrafluoroaluminate, phosphate, orthosilicate, and sulfate. It forms a series of compounds. The ion is toxic to mammals as it causes blockage to iodine uptake in the thyroid. However it is degraded in the body over several hours.
Compounds containing difluorophosphate may have it as a simple uninegative ion, it may function as a difluorophosphato ligand where it is covalently bound to one or two metal atoms, or go on to form a networked solid. It may be covalently bound to a non metal or an organic moiety to make an ester or an amide.
Formation
Ammonium difluorophosphate ([NH4]PO2F2) is formed from treating phosphorus pentoxide with ammonium fluoride. This was how the ion was first made by its discoverer, Willy Lange, in 1929.
Alkali metal chlorides can react with dry difluorophosphoric acid to form alkali metal salts.
- NaCl + HPO2F2 → NaPO2F2 + HCl(g)
Fluorination of dichlorophosphates can produce difluorophosphates. Another method is fluorination of phosphates or polyphosphates.
Trimethylsilyl difluorophosphate ((CH3)3Si−O−P(=O)F2) reacts with metal chlorides to give difluorophosphates.
The anhydride of difluorophosphoric acid (HPO2F2), phosphoryl difluoride oxide (P2O3F4) reacts with oxides such as UO3 to yield difuorophosphates. Phosphoryl difluoride oxide also reacts with alkali metal fluorides to yield difluorophosphates.
Properties
The difluorophosphate ion in ammonium difluorophosphate and potassium difluorophosphate has these interatomic dimensions:
| Difluorophosphate salt | P–O length | P–F length | O–P–O angle | F–P–O angle | F–P–F angle |
|---|---|---|---|---|---|
| Ammonium difluorophosphate | 1.457 Å | 1.541 Å | 118.7° | 109.4° | 98.6° |
| Potassium difluorophosphate | 1.470 Å | 1.575 Å | 122.4° | 108.6° | 97.1° |
Hydrogen bonding from ammonium ion to oxygen atoms causes a change to the difluorophosphate ion in the ammonium salt.
On heating the salts that are not of alkali metals or alkaline earth metals, difluorophosphates decompose firstly by giving off POF3 forming a monofluorophosphate (PO3F) compound, and then this in turn decomposes to an orthophosphate PO3−4 compound.
Difluorophosphate salts are normally soluble and stable in water. However, in acidic or alkaline conditions they can be hydrolyzed to monofluorophosphates and hydrofluoric acid. The caesium and potassium salts are the least soluble.
Irradiating potassium difluorophosphate with gamma rays can make the free radicals •PO2F, •PO3F and •PO2F2.
Compounds
| Formula | Name | Structure | Infrared spectrum | Melting point | Comments | Reference |
|---|---|---|---|---|---|---|
| LiPO2F2 | Lithium difluorophosphate | 360 °C | ||||
| Be(PO2F2)2 | Beryllium difluorophosphate | >400 °C d | prepared from BeCl2 and acid | |||
| CH3CH2−O−P(=O)F2 | Ethyl difluorophosphate | |||||
| [NH4]PO2F−2 | Ammonium difluorophosphate | orthorhombic: a = 8.13 Å, b = 6.43 Å, c = 7·86 Å, Z = 4 space group Pnma | P–F stretching 842 and 860 cm; P–O stretching 1138 and 1292 cm | 213 °C | ||
| [NO2]PO2F−2 | Nitronium difluorophosphate | 515, 530, 550, 560, 575, 845, 880, 1145, 1300, 2390, 3760 cm | nitronium formed from anhydride and N2O5 | |||
| [NO]PO2F−2 | Nitrosonium difluorophosphate | 500, 840, 880, 1130, 1272, 1315, 2278 cm | nitrosonium formed from anhydride and N2O3 | |||
| NaPO2F2 | Sodium difluorophosphate | 210 °C | ||||
| Mg(PO2F2)2 | Magnesium difluorophosphate | 200 °C | ||||
| [NH4]Mg(PO2F−2)3 | Ammonium magnesium difluorophosphate | Cmcm a=5.411 b=15.20 c=12.68 | ||||
| Al(PO2F2)3 | Aluminium difluorophosphate | polymeric | 505, 541, 582, 642, 918, 971, 1200, 1290 cm (with 355 cm impurity) | formed from Al(CH2CH3)3 and acid; colourless insoluble powder | ||
| Si(−O−P(=O)F2)4 | Silicon(IV) difluorophosphate | formed from SiCl4 and anhydride | ||||
| (CH3)3Si−O−P(=O)F2 | Trimethylsilyl difluorophosphate | formed from anhydride and [(CH3)3Si]2O | ||||
| KPO2F2 | Potassium difluorophosphate | orthorhombic: a = 8.03 Å, b = 6.205 Å, c = 7.633 Å, Z = 4, V=380.9 Å, density = 2.44 g/cm | 510, 525, 570, 835, 880, 1145, 1320, 1340 cm | 263 °C | colourless elongated prisms | |
| (K)4(PO2F−2)2(S2O2−7) | Tetrapotassium difluorophosphate pyrosulfate | C2/c: a = 13.00 Å, b = 7.543 Å, c = 19.01 Å, β = 130.07°, Z = 4 | ||||
| Ca(PO2F2)2·CH3COOCH2CH3 | Calcium difluorophosphate - ethyl acetate 1:1 solvate | |||||
| Ca(PO2F2)2 | Calcium difluorophosphate | >345 °C d | ||||
| [VO2]PO2F−2 | Pervanadyl difluorophosphate | |||||
| CrO2(PO2F2)2 | Chromyl difluorophosphate | formed from anhydride; red-brown | ||||
| Cr(PO2F2)3 | Chromium(III) difluorophosphate | 320, 385, 490, 575, 905, 955, 1165, 1255 cm | formed from excess anhydride, green | |||
| Mn(CO)5PO2F2 | 184 °C | |||||
| HMn(PO2F2)3 | dissolve manganese in acid; white | |||||
| [NH+4](Mn)3(PO2F−2)(PO3F)2(F)2 | ||||||
| Fe(PO2F2)2 | Iron(II) difluorophosphate | 463, 496, 668 (weak), 869 (double), 1139, 1290 cm | 180 °C d | colour blue green, hygroscopic, melts 250 °C, above 300 °C starts decomposing to Fe3(PO4)2 | ||
| Fe(PO2F2)3 | Iron(III) difluorophosphate | 262, 493, 528, 570, 914, 965, 1173, 1242 cm | >400 °C | decomposes at 230 °C yielding FeF3; dissolve iron in acid in presence of oxygen | ||
| K(Fe)3(PO2F−2)(PO3F)2(F)2 | ||||||
| Co(PO2F2)2 | Cobalt(II) difluorophosphate | 173 °C | prepared from CoCl2 and acid; pink or blue; blue formed by heating pink to 140 °C | |||
| HCo(PO2F2)3 | dissolve cobalt in acid; red-purple | |||||
| Co(PO2F2)2·2CH3CN | Cobalt(II) difluorophosphate - methyl cyanide solvate 1:2 | orthorhombic: a = 9.227 Å, b = 13.871 Å, c = 9.471 Å, V = 1212 Å, Z = 4, density = 1.88 g/cm | treat HCo(PO2F2)3 with CH3CN for a few weeks; red crystals | |||
| [NH+4](Co)3(PO2F−2)(PO3F)2(F)2 | ||||||
| Ni(PO2F2)2 | Nickel(II) difluorophosphate | 255 °C d | slowly prepared from NiCl2 and acid; yellow | |||
| HNi(PO2F2)3 | dissolve nickel in acid; yellow | |||||
| Cu(PO2F2)2 | Copper(II) difluorophosphate | orthorhombic Fddd: a = 10.134 Å, b = 24.49 Å, c = 34.06 Å, Z = 48, V = 8454.3 Å, density = 2.50 g/cm | 265 °C d | pale blue needles | ||
| CuI(xantphos)2(μ-PO2F2) | polymeric; monoclinic: a = 12.435 Å, b = 10.887 Å, c = 25.682 Å, β = 100.220°, V = 3421 Å | colourless | ||||
| Zn(PO2F2)2 | Zinc(II) difluorophosphate | c. 25 °C? | glassy | |||
| H2[Zn(PO2F2)4] | Tetra(difluorophosphato)zincic(II) acid | |||||
| Ga(PO2F2)3 | Gallium(III) difluorophosphate | |||||
| [(CH3)2GaPO2F2]2 | Dimethylgallium(III) difluorophosphate | dimeric | 380, 492, 520, 551, 616, 709, 750, 899, 949, 1171, 1218, 1262, 1295, 1404, 2922, 2982 cm | |||
| RbPO2F2 | Rubidium difluorophosphate | orthorhombic: a = 8.15 Å, b = 6.45 Å, c = 7.79 Å, Z = 4, V = 409.5 Å density = 3.02 g/cm | P–F stretching 827 and 946 cm; P–O stretching 1145 and 1320 cm | 160 °C | white | |
| Sr(PO2F2)2 | Strontium difluorophosphate | 250 °C d | prepared from SrCl2 and acid | |||
| [NH4]Sr(PO2F2)3 | Ammonium strontium difluorophosphate | Triclinic P1 a=7.370 b=11.054 c=13.645 α=88.861 β=87.435° γ=89.323° | ||||
| AgPO2F2 | Silver(I) difluorophosphate | |||||
| Ag9(PO2F2)14 | ||||||
| Ag(1-methyl-2-alkylthiomethyl-1H-benzimidazole)PO2F2 | ||||||
| Ag(2,6-bis-pyridine)PO2F2 | Triclinic P1: a = 7.687 Å, b = 10.740 Å, c = 13.568 Å, α = 99.52°, β = 96.83°, γ = 99.83°, Z = 2, V = 1076 Å, density = 1.81 g/cm | |||||
| Ag(4,4′-dicyanodiphenylacetylene)PO2F2 | ||||||
| Cd(PO2F2)2 | Cadmium(II) difluorophosphate | 245 °C d | ||||
| In(PO2F2)3 | Indium(III) difluorophosphate | 269, 492, 528, 567, 910, 962, 1179, 1269 cm | white, decomposes at 260 °C yielding InF3 | |||
| [(CH3)2InPO2F2]2 | Dimethylindium(III) difluorophosphate | dimeric | 373, 490, 500, 535, 559, 735, 878, 925, 1128, 1179, 1275, 1435, 2928, 3000 cm | |||
| SnCl2(PO2F2)2 | Tin(IV) dichloride difluorophosphate | |||||
| (CH3)2Sn(PO2F2)2 | Dimethyltin(IV) difluorophosphate | 204 °C d | prepared from (CH3)2SnCl2 and acid; yellow | |||
| (CH3CH2)2Sn(PO2F2)2 | Diethyltin(IV) difluorophosphate | 262 °C d | prepared from (CH3CH2)2SnCl2 and acid; yellow | |||
| (CH3CH2CH2)2Sn(PO2F2)2 | Dipropyltin(IV) difluorophosphate | 245 °C d | prepared from (CH3CH2CH2)2SnCl2 and acid; yellow | |||
| (CH3(CH2)3)2Sn(PO2F2)2 | Dibutyltin(IV) difluorophosphate | 235 °C d | prepared from (CH3(CH2)7)2SnCl2 and acid; yellow | |||
| (CH3(CH2)7)2Sn(PO2F2)2 | Dioctyltin(IV) difluorophosphate | 114 °C | prepared from (CH3(CH2)7)2SnCl2 and acid; yellow | |||
| SbCl4PO2F2 | Antimony(V) tetrachloride difluorophosphate | |||||
| SbF4PO2F2 | Antimony(V) tetrafluoride difluorophosphate | |||||
| (2,2-dipyradyl)2Re(CO)2PO2F2 | ||||||
| AuPO2F2 | ||||||
| IO2PO2F2 | Raman: 130, 163, 191, 219, 295, 323, 329, 378, 637, 713, 737, 781, 799, 839, 918, 1163 cm | yellowish colour, produced from IO3, decomposed by water | ||||
| IO3PO2F2 | Raman: 217, 247, 269, 305, 343, 367, 395, 473, 569, 643, 671, 717, 797, 891, 1123 cm | yellowish colour, produced from H5IO6, decomposed by water | ||||
| FXePO2F2 | Xenon(II) fluoride difluorophosphate | |||||
| Xe(PO2F2)2 | Xenon(II) difluorophosphate | |||||
| CsPO2F2 | Caesium difluorophosphate | orthorhombic: a = 8.437 Å, b = 6.796 Å, c = 8.06 Å, Z = 4, V = 462.1 Å, density = 3.36 g/cm | 286 °C | |||
| (Cs)2(Fe)2(PO2F−2)(PO3F)2(F)3 | ||||||
| Ba(PO2F2)2 | Barium difluorophosphate | orthorhombic I42d a =10.4935 b =10.4935 c =26.030 | >400 °C | |||
| [NH4]2Ba(PO2F2)4 | Diammonium barium difluorophosphate | P2/n a=14.285 b=5.472 c=19.474 β=97.607° | ||||
| Re(CO)5PO2F2 | ||||||
| Hg(PO2F2)2 | Mercury(II) difluorophosphate | |||||
| Hg2(PO2F2)2 | Mercury(I) difluorophosphate or di(difluorophosphato)dimercurane | Raman: 220 cm | produced from anhydride | |||
| TlPO2F2 | Thallium(I) difluorophosphate | produced from anhydride, or acid on TlCl | ||||
| [(CH3)2TlPO2F2]2 | Dimethylthallium(III) difluorophosphate | dimeric | 360, 374, 500, 505, 520, 559, 850, 880, 1120, 1140, 1195, 1250, 1285, 2932, 3020 cm | |||
| Pb(PO2F2)2 | Lead(II) difluorophosphate | 189 °C d | ||||
| UO2(PO2F2)2 | Uranyl difluorophosphate | 260, 498, 854, 924, 980, 1124 cm | IR spectrum due to UO2+2 | |||
| [(CH3CH2)4N]PO2F−2 | Tetraethylammonium difluorophosphate | |||||
| 1-ethyl-3-methylimidazolium difluorophosphate | ionic liquid | |||||
| 1-butyl-3-methylimidazolium difluorophosphate | ionic liquid | |||||
| 1-butyl-1-methylpyrrolidinium difluorophosphate | ionic liquid | |||||
| 1-butyl-1-methylpiperidinium difluorophosphate | ionic liquid | |||||
| di(3,3′,4,4′-tetramethyl-2,2′,5,5′-tetraselenafulvalenium)difluorophosphate | Transitions to a metallic state below 137 K (−136 °C) | |||||
| 1,4-diphenyl-3,5-enanilo-4,5-dihydro-1,2,4-triazole (nitron) | monoclinic P21/n: a = 7.3811 Å, b = 14.9963 Å, c = 16.922 Å, β = 102.138°, V = 1361.2 Å, Z = 4 | insoluble; yellow-brown | ||||
| Strychnine PO2F2 | ||||||
| Cocaine PO2F2 | ||||||
| Brucine PO2F2 | ||||||
| Morphine PO2F2 | ||||||
| [N(CH3)4]PO2F−2 | Tetramethylammonium difluorophosphate | |||||
| H[B(PO2F2)4] | Tetra(difluorophosphato)boric acid | 469, 502, 552, 647, 836, 940, 994, 1093, 1348, 1567 cm | formed from BBr3 and acid; liquid | |||
| Li[B(PO2F2)4] | Lithium tetra(difluorophosphato)borate | monoclinic P21/c: a=7.9074 Å, b = 14.00602 Å, c = 13.7851 Å, β = 121.913°, Z = 4 | 479, 502, 568, 833, 945, 1002, 1080, 1334 cm | formed from HB(PO2F2)4 and butyllithium; colourless | ||
| [HS(CH3)2][B(PO2F2)4] | Dimethylsulfonium tetra(difluorophosphato)borate | 472, 511, 555, 648, 832, 933, 993, 1082, 1337, 1436, 2851, 2921, 3042 cm | formed from BH3·S(CH3)2 and acid; ionic liquid | |||
| [Li((CH3CH2)2O)]3[Al(PO2F2)6] | (Diethyl ether)lithium hexa(difluorophosphato)aluminate | trigonal R3: a = 17.4058 Å, b = 17.4058 Å, c = 21.4947 Å, γ = 120°, Z = 6 | 417, 503, 536, 624, 723, 891, 922, 964, 1174, 1204, 1283 cm | formed from butyllithium and triethylaluminium and the acid; white | ||
| K2CrO2(PO2F2)4 | 305, 370, 485, 550, 870, 920, 1050, 1130, 1250 cm | 145 °C d | formed from anhydride and K2CrO4; brown | |||
| Na2MoO2(PO2F2)4 | amorphous | 280, 490, 620, 880, 915, 950, 1020, 1070, 1140, 1280 cm | 125 °C d | formed from anhydride and K2MoO4; white | ||
| Na2WO2(PO2F2)4 | amorphous | 280, 474, 620, 930, 1030, 1130, 1230 cm | 109 °C d | formed from anhydride and K2WO4; white |
Related substances
Difluorphosphoric acid
Difluorophosphoric acid (HPO2F2) is one of the fluorophosphoric acids. It is produced when phosphoryl fluoride reacts with water:
- POF3 + H2O → HPO2F2 + HF
This in turn is hydrolysed more to give monofluorophosphoric acid (H2PO3F), and a trace of hexafluorophosphoric acid (HPF6). HPO2F2 also is produced when HF reacts with phosphorus pentoxide. Yet another method involves making difluorphosphoric acid as a side product of calcium fluoride being heated with damp phosphorus pentoxide. A method to make pure difluorphosphoric acid involves heating phosphoryl fluoride with monofluorophosphoric acid and separating the product by distillation:
- POF3 + H2PO3F → 2 HPO2F2
Difluorophosphoric acid can also be produced by fluorinating phosphorus oxychlorides. P2O3Cl4 and POCl3 react with hydrogen fluoride solution to yield HPO2Cl2 and then HPO2F2. Yet another way is to treat orthophosphate (PO3−4) with fluorosulfuric acid (HSO3F).
Difluorphosphoric acid is a colorless liquid. It melts at −96.5 °C (−141.7 °F) and boils at 115.9 °C (240.6 °F). Its density at 25 °C is 1.583 g/cm.
Phosphoryl difluoride oxide
Difluorophosphoric acid anhydride also known as phosphoryl difluoride oxide or diphosphoryl tetrafluoride (F2(O=)P−O−P(=O)F2 or P2O3F4) is an anhydride of difluorphosphoric acid. It crystallises in the orthorhombic system, with space group Pcca and Z = 4. P2O3F4 can be made by refluxing difluorophosphoric acid with phosphorus pentoxide. P2O3F4 boils at 71 °C.
Substitution
In addition to the isoelectronic series, ions related by substituting fluorine or oxygen by other elements include monofluorophosphate, difluorothiophosphate, dichlorothiophosphate, dichlorophosphate, chlorofluorothiophosphate, chlorofluorophosphate, dibromophosphate, and bromofluorophosphate.
Adducts
Difluorophosphate can form adducts with PF5 and AsF5. In these the oxygen atoms form a donor-acceptor link between the P and As (or P) atoms, linking the difluorides to the pentafluorides. Example salts include KPO2F2·2AsF5, KPO2F2·AsF5, KPO2F2·2PF5 and KPO2F2·PF5.
Amines can react with phosphoryl fluoride to make substances with a formula RR′N−P(=O)F2. The amines shown to do this include ethylamine, isopropylamine, n-butylamine, t-butylamine, dimethylamine, and diethylamine. The monoamines can further react to yield an alkyliminophosphoric fluoride (R−N=P(=O)F).
References
- Toy, Arthur D. F. (22 Oct 2013). The Chemistry of Phosphorus. Pergamon Texts in Inorganic Chemistry. Vol. 3. Pergamon Press. pp. 536–537. ISBN 9781483147413. Retrieved 19 June 2015.
- ^ Anbar, M.; Guttmann, S.; Lewitus, Z. (30 May 1959). "Effect of Monofluorosulphonate, Difluorophosphate and Fluoroborate Ions on the Iodine Uptake of the Thyroid Gland". Nature. 183 (4674): 1517–1518. Bibcode:1959Natur.183.1517A. doi:10.1038/1831517a0. PMID 13666792. S2CID 4291858.
- ^ Lange, Willy (3 April 1929). "Über die Difluorphosphorsäure und ihre der Perchlorsäure ähnliche Salzbildung" [On difluorophosphoric acid and its perchlorate-like salt formation]. Berichte der Deutschen Chemischen Gesellschaft (A and B Series). 62 (4): 786–792. doi:10.1002/cber.19290620408.
- ^ Schulz, Christoph; Eiden, Philipp; Klose, Petra; Ermantraut, Andreas; Schmidt, Michael; Garsuch, Arnd; Krossing, Ingo (2015). "Homoleptic borates and aluminates containing the difluorophosphato ligand – [M(O2PF2)x] – synthesis and characterization". Dalton Trans. 44 (15): 7048–7057. doi:10.1039/c5dt00469a. PMID 25785817.
- ^ Vast, P.; Semmoud, A.; Addou, A.; Palavit, G. (March 1988). "Étude méthodologique de la synthèse des difluorodioxophosphates métalliques à partir de l'oxyde difluorure de phosphoryle" [Methodical study of the synthesis of metal difluorodioxophosphates from phosphoryl difluoride oxide]. Journal of Fluorine Chemistry. 38 (3): 297–302. doi:10.1016/S0022-1139(00)81065-9.
- ^ Thompson, R.C.; Reed, W. (July 1969). "Preparation and infrared spectra of alkali metal difluorophosphates". Inorganic and Nuclear Chemistry Letters. 5 (7): 581–585. doi:10.1016/0020-1650(69)80034-6.
- ^ Weidlein, J. (April 1968). "Darstellung, Eigenschaften und IR-Spektren von OTi(O2PCl2)2, Fe(O2PF2)3 und In(O2PF2)3" [Description, properties and IR spectra of...]. Zeitschrift für anorganische und allgemeine Chemie. 358 (1–2): 13–20. doi:10.1002/zaac.19683580103.
- ^ Shihada, Abdel-Fattah; Mohammed, Abdulalah T. (1 January 1980). "Zur Reaktion von Trimethylsilyl-difluorophosphat, Trimethylsilyl-dimethylphosphinat und Difluorophosphorsäureanhydrid mit TiCl4 bzw. SbCl5" [On the Reactions of Trimethylsilyl Difluorophosphate, Trimethylsilyl Dimethylphosphinate and Difluorophosphoric Acid Anhydride with TiCl4 and SbCl5]. Zeitschrift für Naturforschung B. 35 (1): 60–63. doi:10.1515/znb-1980-0114. S2CID 96302051.
- ^ Vast, P.; Semmoud, A. (January 1985). "Préparation de nouveaux difluorodioxophosphates à partir de l'oxyde de difluorure de phosphoryle. Partie V. Réactions sur le trioxyde d'uranium" [Preparation of new difluorodioxophosphates from phosphoryl difluorideoxide. Part V. Reactions with uranium dioxide]. Journal of Fluorine Chemistry. 27 (1): 47–52. doi:10.1016/S0022-1139(00)80896-9.
- Addou, A.; Vast, P.; Legrand, P. (January 1982). "Champ de forces de symetrie locale des composés oxyfluorés du phosphore(V). I. Les difluorodioxophosphates (DFP) alcalins" [Local symmetry force-field of oxyfluorine compounds of phosphorus(V). I. Alkali difluorodioxophosphates (DFP)]. Spectrochimica Acta Part A: Molecular Spectroscopy. 38 (7): 785–790. Bibcode:1982AcSpA..38..785A. doi:10.1016/0584-8539(82)80068-8.
- ^ Harrison, R. W.; Trotter, James (1969). "Structure of ammonium difluorophosphate". Journal of the Chemical Society A: Inorganic, Physical, Theoretical: 1783. doi:10.1039/J19690001783.
- ^ Vast, P.; Semmoud, A. (June 1994). "Comportement thermique du difluorodioxophosphate ferreux" [Thermal behaviour of ferrous difluorodioxophosphate]. Journal of Thermal Analysis. 41 (6): 1489–1493. doi:10.1007/bf02549945. S2CID 95079191.
- Vast, P.; Semmoud, A.; Palavit, G. (December 1986). "Préparation de l'acide monofluorotrioxophosphorique H2PO3F à partir de l'acide difluorodioxophosphorique HPO2F2" [Preparation of monofluorotrioxophosphoricacid H2PO3F from difluorodioxophosphoric acid HPO2F2]. Journal of Fluorine Chemistry. 34 (2): 229–232. doi:10.1016/s0022-1139(00)85072-1.
- ^ Reed, William (September 1965). Studies of Difluorophosphoric Acid and its Alkali Metal Salts (Thesis). Retrieved 23 April 2023.
- Begum, Afrozi; Subramanian, S.; Symons, M. C. R. (1970). "Unstable intermediates. Part LXVIII. Electron spin resonance studies of the radicals O3PF and O2PF2". Journal of the Chemical Society A: Inorganic, Physical, Theoretical: 1323. doi:10.1039/J19700001323.
- Begum, A.; Subramanian, S.; Symons, M. C. R. (1971). "Unstable intermediates. Part LXXXVI. Electron spin resonance studies of the effect of γ-rays on potassium difluorophosphate: the radical PO2F?". Journal of the Chemical Society A: Inorganic, Physical, Theoretical: 700–702. doi:10.1039/J19710000700.
- ^ Tan, Thiam Hock (August 1970). Preparation and Properties of Metal Difluorophosphates (Thesis). University of British Columbia. Archived from the original (PDF) on 2016-03-04.
- ^ Roesky, Herbert W. (July 1967). "Über Reaktionen mit Pyrophosphoryltetrafluorid" [On Reactions with pyrophosphoryl tetrafluoride]. Chemische Berichte. 100 (7): 2147–2150. doi:10.1002/cber.19671000706.
- ^ Addou, A.; Vast, P. (August 1979). "Préparation de nouveaux difluorodioxophosphates à partir de l'oxyde de difluororure de phosphoryle. Partie I. Réactions sur les oxydes d'azote" [Preparation of new difluorodioxophosphates from phosphoryl difluoride oxide. Part I. Reactions with nitrogen oxides]. Journal of Fluorine Chemistry. 14 (2): 163–169. doi:10.1016/S0022-1139(00)82884-5.
- ^ Zhang, Wenyao; Jin, Wenqi; Cheng, Meng; Zhang, Ruonan; Yang, Zhihua; Pan, Shilie (2021). "From centrosymmetric to noncentrosymmetric: effect of the cation on the crystal structures and birefringence values of (NH 4 ) n−2 AE(PO 2 F 2 ) n (AE = Mg, Sr and Ba; n = 2, 3 and 4)". Dalton Transactions. 50 (29): 10206–10213. doi:10.1039/D1DT00698C. ISSN 1477-9226. PMID 34231608. S2CID 235758275.
- ^ Trotter, James; Whitlow, S. H. (1967). "The structures of caesium and rubidium difluorophosphates". Journal of the Chemical Society A: Inorganic, Physical, Theoretical: 1383–1386. doi:10.1039/J19670001383.
- Harrison, R. W.; Thompson, R. C.; Trotter, James (1966). "The structure of potassium difluorophosphate". Journal of the Chemical Society A: Inorganic, Physical, Theoretical: 1775. doi:10.1039/J19660001775.
- Zhang, Wenyao; Jin, Wenqi; Yang, Zhihua; Pan, Shilie (2020). "K4(PO2F2)2(S2O7): first fluorooxophosphorsulfate with mixed-anion [S2O7] and [PO2F2] groups". Dalton Transactions. 49 (48): 17658–17664. doi:10.1039/D0DT03307C. ISSN 1477-9226. PMID 33231582. S2CID 227157666.
- Grunze, H.; Jost, K.-H.; Wolf, G.-U. (April 1969). "Salze von Halogenophosphorsäuren. V. Darstellung und Struktur des Bis(äthylacetat)-calcium-difluorophosphates Ca2 · 2 CH3COOC2H5" [Salts of halophosphoric acids. V. Description and structure of bis(ethylacetate)calcium difluorophosphate...]. Zeitschrift für anorganische und allgemeine Chemie. 365 (5–6): 294–300. doi:10.1002/zaac.19693650509.
- ^ Brown, S.D.; Emme, L.M.; Gard, G.L. (December 1975). "The reaction of chromium trioxide and metal oxide salts with P2O3F4". Journal of Inorganic and Nuclear Chemistry. 37 (12): 2557–2558. doi:10.1016/0022-1902(75)80891-8.
- Wimmer, F.L.; Snow, M.R. (1978). "Perchlorate and difluorophosphate coordination derivatives of manganese carbonyl". Australian Journal of Chemistry. 31 (2): 267. doi:10.1071/CH9780267.
- ^ Dove, Michael F. A.; Hibbert, Richard C.; Logan, Norman (1985). "Difluorophosphate complexes of chromium, manganese, iron, cobalt, and nickel". Journal of the Chemical Society, Dalton Transactions (4): 707. doi:10.1039/DT9850000707.
- ^ Weil, Matthias; Fürst, Markus (2020-07-01). "Crystal structure of (1,4-diphenyl-4H-1,2,4-triazol-3-yl)phenylamine difluorophosphate, and a survey of the difluorophosphate anion (PO2F2)". Acta Crystallographica Section E. 76 (7): 1003–1006. doi:10.1107/S2056989020006933. ISSN 2056-9890. PMC 7336792. PMID 32695441.
- ^ Begley, Michael J.; Dove, Michael F. A.; Hibbert, Richard C.; Logan, Norman; Nunn, Michael; Sowerby, D. Bryan (1985). "Crystal structures of the difluorophosphate complexes, Co(O2PF2)2 · 2MeCN and Cu(O2PF2)2". Journal of the Chemical Society, Dalton Transactions (11): 2433–2436. doi:10.1039/DT9850002433.
- Keller, Sarah; Brunner, Fabian; Prescimone, Alessandro; Constable, Edwin C.; Housecroft, Catherine E. (August 2015). "Hexafluoridophosphate partial hydrolysis leading to the one-dimensional coordination polymer ". Inorganic Chemistry Communications. 58: 64–66. doi:10.1016/j.inoche.2015.06.002.
- ^ Schaible, B.; Weidlein, J. (February 1974). "Untersuchungen an Dialkylmetallphosphor- und -phosphinsäurederivaten der Elemente Aluminium, Gallium, Indium und Thallium. II. Die Schwingungsspektren der Difluoro- und Dichlorophosphate" [Investigations upon dialkylmetal phosphate and phosphite derivatives of the elements aluminium, gallium, indium and thallium. II. The vibrational spectroscopy of difluoro- and dichlorophosphates]. Zeitschrift für anorganische und allgemeine Chemie. 403 (3): 301–309. doi:10.1002/zaac.19744030309.
- ^ Albrecht, Markus; Hübler, Klaus; Kaim, Wolfgang (May 2000). "Syntheses, Structures, and Properties of the Complexes (X), N∧S = 1-Methyl-2-(alkylthiomethyl)-1H-benzimidazoles, X = PF6 (n = 2) or PO2F2 (n = 1)". Zeitschrift für anorganische und allgemeine Chemie. 626 (5): 1033–1037. doi:10.1002/(SICI)1521-3749(200005)626:5<1033::AID-ZAAC1033>3.0.CO;2-A.
- Fessler, Th. U.; Hübener, R.; Strähle, J. (September 1997). "Synthese von Kupfer- und Silberkomplexen mit fünfzähnigen N,S- und sechszähnigen N,O-Chelatliganden – Charakterisierung und Kristallstrukturen von {Cu22(C5H5N)4}, {Cu22}(PF6)2 und {Ag}PO2F2" [Synthesis of copper und silver complexes with pentadentate N,S- und hexadentate N,O-chelation ligands – characterization und crystal structures of...]. Zeitschrift für anorganische und allgemeine Chemie. 623 (9): 1367–1374. doi:10.1002/zaac.19976230908.
- ^ Krüger, N.; Dehnicke, K.; Shihada, A.-F. (February 1978). "Difluorophosphate von Zinn(IV) und Antimon(V)" [Difluorophosphates of tin(IV) and antimony(V)]. Zeitschrift für anorganische und allgemeine Chemie. 438 (1): 169–175. doi:10.1002/zaac.19784380118.
- ^ Horn, E.; Snow, M.R. (1980). "Perchlorate and difluorophosphate coordination derivatives of rhenium carbonyl". Australian Journal of Chemistry. 33 (11): 2369. doi:10.1071/CH9802369.
- LeBlanc, D. J.; Britten, J. F.; Lock, C. J. L. (15 September 1997). "Bis(triphenylphosphine sulfide-)gold(I) Difluorophosphate(V)". Acta Crystallographica Section C. 53 (9): 1204–1206. doi:10.1107/S0108270197005209.
- ^ Addou, A.; Vast, P. (July 1980). "Préparation de nouveaux difluorodioxophosphates à partir de l'oxyde de difluorure de phosphoryle. Partie II. Réactions sur les acides iodique et periodique" [Preparation of new difluorodioxophosphates from phosphoryl difluoride oxide. Part II. Reactions with iodic and periodic acids]. Journal of Fluorine Chemistry. 16 (1): 89–96. doi:10.1016/s0022-1139(00)85151-9.
- ^ Eisenberg, Max; Desmarteau, Darryl D. (August 1972). "Xenon(II) difluorophosphates. Preparations, properties, and evidence for the difluorophosphate free radical". Inorganic Chemistry. 11 (8): 1901–1904. doi:10.1021/ic50114a033.
- Matsumoto, Kazuhiko; Okawa, Takeshi; Hagiwara, Rika (2012). "The Crystal to Plastic Crystal Phase Transition of Tetraethylammonium Difluorophosphate and Tetrafluoroborate". Chemistry Letters. 41 (4): 394–396. doi:10.1246/cl.2012.394. hdl:2433/259816. S2CID 97011854.
- ^ Matsumoto, Kazuhiko; Hagiwara, Rika (3 August 2009). "A New Series of Ionic Liquids Based on the Difluorophosphate Anion". Inorganic Chemistry. 48 (15): 7350–7358. doi:10.1021/ic9008009. PMID 19580312.
- Eriks, K.; Wang, H. H.; Reed, P. E.; Beno, M. A.; Appelman, E. H.; Williams, J. M. (15 February 1985). "Di(3,3′,4,4′-tetramethyl-2,2′,5,5′-tetraselenafulvalenium)difluorophosphate, (C10H12Se4)2PO2F2, at 293 and 125 K". Acta Crystallographica Section C. 41 (2): 257–260. doi:10.1107/S0108270185003535.
- Lange, Willy; Livingston, Ralph (March 1950). "Studies of Fluorophosphoric Acids and their Derivatives. XIV. Preparation of Anhydrous Difluorophosphoric Acid". Journal of the American Chemical Society. 72 (3): 1280–1281. doi:10.1021/ja01159a057.
- Semmoud, A.; Benghalem, A.; Addou, A. (January 1990). "Acide difluorophosphorique: nouvelle préparation" [Difluorophosphoric acid: new preparation]. Journal of Fluorine Chemistry. 46 (1): 1–6. doi:10.1016/S0022-1139(00)81555-9.
- Vast, P.; Semmoud, A.; Addou, A.; Palavit, G. (March 1985). "Nouvelle méthode de preparation de l'acide difluorophosphorique" [New preparation method of difluorophosphoric acid]. Journal of Fluorine Chemistry. 27 (3): 319–325. doi:10.1016/S0022-1139(00)81312-3.
- Zeng, Xiaoqing; Gerken, Michael; Beckers, Helmut; Willner, Helge (17 June 2010). "ChemInform Abstract: Spectroscopic and Structural Studies of Difluorophosphoryl Azide F2P(O)N3, Difluorophosphoryl Isocyanate F2P(O)NCO, and Difluorophosphoric Acid Anhydride, F2(O)POP(O)F2". ChemInform. 41 (28): no. doi:10.1002/chin.201028001. Originally in Inorg. Chem. 49 (2010) 6, 3002–3010
- Robinson, E. A. (September 1962). "The Preparation and Raman Spectrum of Diphosphoryl Tetrafluoride: Comparison with the Spectrum of Diphosphoryl Tetrachloride". Canadian Journal of Chemistry. 40 (9): 1725–1729. doi:10.1139/v62-264.
- Dehnicke, Kurt; Shihada, Abdel-Fattah (1976). "Structural and bonding aspects in phosphorus chemistry—inorganic derivatives of oxohalogeno phosphoric acids". Electrons in Oxygen- and Sulphur-Containing Ligands Structure and Bonding. Structure and Bonding. 28: 51–82. doi:10.1007/3-540-07753-7_2. ISBN 978-3-540-07753-4.
- Christe, K. O.; GNANN, R.; WAGNER, R. I.; WILSON, W. W. (4 August 2010). "ChemInform Abstract: The (PO2F2×2 AsF5) Anion, an Example of a Stable, Oxygen- Bridged, 1:2 Donor-Acceptor Polynuclear Anion". ChemInform. 28 (2): no. doi:10.1002/chin.199702025. Full article at Eur. J. Solid State Inorg. Chem. 33 (1996) 9, 865–877
- Olah, Georg; Oswald, Alexius; Kuhn, Stephan (4 August 1959). "Untersuchung organischer Phosphorverbindungen. III. Darstellung von Difluorphosphorsäure- und von Difluorthiophosphorsäure-alkylamiden" [Investigations of organic phosphorus compounds. III. description of difluorophosphoric and difluorothiophosphoric alkylamides]. Justus Liebigs Annalen der Chemie. 625 (1): 88–91. doi:10.1002/jlac.19596250111.

