In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in their reference state, with all substances in their standard states. The standard pressure value p = 10 Pa (= 100 kPa = 1 bar) is recommended by IUPAC, although prior to 1982 the value 1.00 atm (101.325 kPa) was used. There is no standard temperature. Its symbol is ΔfH. The superscript Plimsoll on this symbol indicates that the process has occurred under standard conditions at the specified temperature (usually 25 °C or 298.15 K).
Standard states are defined for various types of substances. For a gas, it is the hypothetical state the gas would assume if it obeyed the ideal gas equation at a pressure of 1 bar. For a gaseous or solid solute present in a diluted ideal solution, the standard state is the hypothetical state of concentration of the solute of exactly one mole per liter (1 M) at a pressure of 1 bar extrapolated from infinite dilution. For a pure substance or a solvent in a condensed state (a liquid or a solid) the standard state is the pure liquid or solid under a pressure of 1 bar.
For elements that have multiple allotropes, the reference state usually is chosen to be the form in which the element is most stable under 1 bar of pressure. One exception is phosphorus, for which the most stable form at 1 bar is black phosphorus, but white phosphorus is chosen as the standard reference state for zero enthalpy of formation.
For example, the standard enthalpy of formation of carbon dioxide is the enthalpy of the following reaction under the above conditions:
All elements are written in their standard states, and one mole of product is formed. This is true for all enthalpies of formation.
The standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole (kJ mol), but also in kilocalorie per mole, joule per mole or kilocalorie per gram (any combination of these units conforming to the energy per mass or amount guideline).
All elements in their reference states (oxygen gas, solid carbon in the form of graphite, etc.) have a standard enthalpy of formation of zero, as there is no change involved in their formation.
The formation reaction is a constant pressure and constant temperature process. Since the pressure of the standard formation reaction is fixed at 1 bar, the standard formation enthalpy or reaction heat is a function of temperature. For tabulation purposes, standard formation enthalpies are all given at a single temperature: 298 K, represented by the symbol ΔfH
298 K.
Hess' law
For many substances, the formation reaction may be considered as the sum of a number of simpler reactions, either real or fictitious. The enthalpy of reaction can then be analyzed by applying Hess' law, which states that the sum of the enthalpy changes for a number of individual reaction steps equals the enthalpy change of the overall reaction. This is true because enthalpy is a state function, whose value for an overall process depends only on the initial and final states and not on any intermediate states. Examples are given in the following sections.
Ionic compounds: Born–Haber cycle
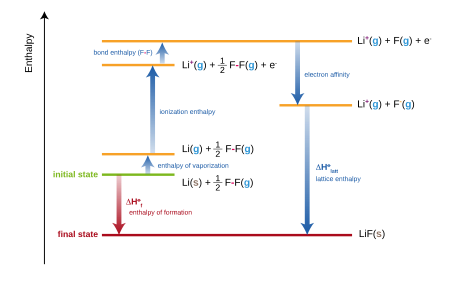
For ionic compounds, the standard enthalpy of formation is equivalent to the sum of several terms included in the Born–Haber cycle. For example, the formation of lithium fluoride,
may be considered as the sum of several steps, each with its own enthalpy (or energy, approximately):
- Hsub, the standard enthalpy of atomization (or sublimation) of solid lithium.
- IELi, the first ionization energy of gaseous lithium.
- B(F–F), the standard enthalpy of atomization (or bond energy) of fluorine gas.
- EAF, the electron affinity of a fluorine atom.
- UL, the lattice energy of lithium fluoride.
The sum of these enthalpies give the standard enthalpy of formation (ΔfH) of lithium fluoride:
In practice, the enthalpy of formation of lithium fluoride can be determined experimentally, but the lattice energy cannot be measured directly. The equation is therefore rearranged to evaluate the lattice energy:
Organic compounds
The formation reactions for most organic compounds are hypothetical. For instance, carbon and hydrogen will not directly react to form methane (CH4), so that the standard enthalpy of formation cannot be measured directly. However the standard enthalpy of combustion is readily measurable using bomb calorimetry. The standard enthalpy of formation is then determined using Hess's law. The combustion of methane:
is equivalent to the sum of the hypothetical decomposition into elements followed by the combustion of the elements to form carbon dioxide (CO2) and water (H2O):
Applying Hess's law,
Solving for the standard of enthalpy of formation,
The value of is determined to be −74.8 kJ/mol. The negative sign shows that the reaction, if it were to proceed, would be exothermic; that is, methane is enthalpically more stable than hydrogen gas and carbon.
It is possible to predict heats of formation for simple unstrained organic compounds with the heat of formation group additivity method.
Use in calculation for other reactions
The standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and products using Hess's law. A given reaction is considered as the decomposition of all reactants into elements in their standard states, followed by the formation of all products. The heat of reaction is then minus the sum of the standard enthalpies of formation of the reactants (each being multiplied by its respective stoichiometric coefficient, ν) plus the sum of the standard enthalpies of formation of the products (each also multiplied by its respective stoichiometric coefficient), as shown in the equation below:
If the standard enthalpy of the products is less than the standard enthalpy of the reactants, the standard enthalpy of reaction is negative. This implies that the reaction is exothermic. The converse is also true; the standard enthalpy of reaction is positive for an endothermic reaction. This calculation has a tacit assumption of ideal solution between reactants and products where the enthalpy of mixing is zero.
For example, for the combustion of methane, :
However is an element in its standard state, so that , and the heat of reaction is simplified to
which is the equation in the previous section for the enthalpy of combustion .
Key concepts for enthalpy calculations
- When a reaction is reversed, the magnitude of ΔH stays the same, but the sign changes.
- When the balanced equation for a reaction is multiplied by an integer, the corresponding value of ΔH must be multiplied by that integer as well.
- The change in enthalpy for a reaction can be calculated from the enthalpies of formation of the reactants and the products
- Elements in their standard states make no contribution to the enthalpy calculations for the reaction, since the enthalpy of an element in its standard state is zero. Allotropes of an element other than the standard state generally have non-zero standard enthalpies of formation.
Examples: standard enthalpies of formation at 25 °C
Thermochemical properties of selected substances at 298.15 K and 1 atm
Inorganic substances
| Species | Phase | Chemical formula | ΔfH /(kJ/mol) |
|---|---|---|---|
| Aluminium | Solid | Al | 0 |
| Aluminium chloride | Solid | AlCl3 | −705.63 |
| Aluminium oxide | Solid | Al2O3 | −1675.5 |
| Aluminium hydroxide | Solid | Al(OH)3 | −1277 |
| Aluminium sulphate | Solid | Al2(SO4)3 | −3440 |
| Barium chloride | Solid | BaCl2 | −858.6 |
| Barium carbonate | Solid | BaCO3 | −1216 |
| Barium hydroxide | Solid | Ba(OH)2 | −944.7 |
| Barium oxide | Solid | BaO | −548.1 |
| Barium sulfate | Solid | BaSO4 | −1473.3 |
| Beryllium | Solid | Be | 0 |
| Beryllium hydroxide | Solid | Be(OH)2 | −903 |
| Beryllium oxide | Solid | BeO | −609.4 |
| Boron trichloride | Solid | BCl3 | −402.96 |
| Bromine | Liquid | Br2 | 0 |
| Bromide ion | Aqueous | Br | −121 |
| Bromine | Gas | Br | 111.884 |
| Bromine | Gas | Br2 | 30.91 |
| Bromine trifluoride | Gas | BrF3 | −255.60 |
| Hydrogen bromide | Gas | HBr | −36.29 |
| Cadmium | Solid | Cd | 0 |
| Cadmium oxide | Solid | CdO | −258 |
| Cadmium hydroxide | Solid | Cd(OH)2 | −561 |
| Cadmium sulfide | Solid | CdS | −162 |
| Cadmium sulfate | Solid | CdSO4 | −935 |
| Caesium | Solid | Cs | 0 |
| Caesium | Gas | Cs | 76.50 |
| Caesium | Liquid | Cs | 2.09 |
| Caesium(I) ion | Gas | Cs | 457.964 |
| Caesium chloride | Solid | CsCl | −443.04 |
| Calcium | Solid | Ca | 0 |
| Calcium | Gas | Ca | 178.2 |
| Calcium(II) ion | Gas | Ca | 1925.90 |
| Calcium(II) ion | Aqueous | Ca | −542.7 |
| Calcium carbide | Solid | CaC2 | −59.8 |
| Calcium carbonate (Calcite) | Solid | CaCO3 | −1206.9 |
| Calcium chloride | Solid | CaCl2 | −795.8 |
| Calcium chloride | Aqueous | CaCl2 | −877.3 |
| Calcium phosphate | Solid | Ca3(PO4)2 | −4132 |
| Calcium fluoride | Solid | CaF2 | −1219.6 |
| Calcium hydride | Solid | CaH2 | −186.2 |
| Calcium hydroxide | Solid | Ca(OH)2 | −986.09 |
| Calcium hydroxide | Aqueous | Ca(OH)2 | −1002.82 |
| Calcium oxide | Solid | CaO | −635.09 |
| Calcium sulfate | Solid | CaSO4 | −1434.52 |
| Calcium sulfide | Solid | CaS | −482.4 |
| Wollastonite | Solid | CaSiO3 | −1630 |
| Carbon (Graphite) | Solid | C | 0 |
| Carbon (Diamond) | Solid | C | 1.9 |
| Carbon | Gas | C | 716.67 |
| Carbon dioxide | Gas | CO2 | −393.509 |
| Carbon disulfide | Liquid | CS2 | 89.41 |
| Carbon disulfide | Gas | CS2 | 116.7 |
| Carbon monoxide | Gas | CO | −110.525 |
| Carbonyl chloride (Phosgene) | Gas | COCl2 | −218.8 |
| Carbon dioxide (un–ionized) | Aqueous | CO2(aq) | −419.26 |
| Bicarbonate ion | Aqueous | HCO3 | −689.93 |
| Carbonate ion | Aqueous | CO3 | −675.23 |
| Monatomic chlorine | Gas | Cl | 121.7 |
| Chloride ion | Aqueous | Cl | −167.2 |
| Chlorine | Gas | Cl2 | 0 |
| Chromium | Solid | Cr | 0 |
| Copper | Solid | Cu | 0 |
| Copper(II) bromide | Solid | CuBr2 | −138.490 |
| Copper(II) chloride | Solid | CuCl2 | −217.986 |
| Copper(II) oxide | Solid | CuO | −155.2 |
| Copper(II) sulfate | Aqueous | CuSO4 | −769.98 |
| Fluorine | Gas | F2 | 0 |
| Monatomic hydrogen | Gas | H | 218 |
| Hydrogen | Gas | H2 | 0 |
| Water | Gas | H2O | −241.818 |
| Water | Liquid | H2O | −285.8 |
| Hydrogen ion | Aqueous | H | 0 |
| Hydroxide ion | Aqueous | OH | −230 |
| Hydrogen peroxide | Liquid | H2O2 | −187.8 |
| Phosphoric acid | Liquid | H3PO4 | −1288 |
| Hydrogen cyanide | Gas | HCN | 130.5 |
| Hydrogen bromide | Liquid | HBr | −36.3 |
| Hydrogen chloride | Gas | HCl | −92.30 |
| Hydrogen chloride | Aqueous | HCl | −167.2 |
| Hydrogen fluoride | Gas | HF | −273.3 |
| Hydrogen iodide | Gas | HI | 26.5 |
| Iodine | Solid | I2 | 0 |
| Iodine | Gas | I2 | 62.438 |
| Iodine | Aqueous | I2 | 23 |
| Iodide ion | Aqueous | I | −55 |
| Iron | Solid | Fe | 0 |
| Iron carbide (Cementite) | Solid | Fe3C | 5.4 |
| Iron(II) carbonate (Siderite) | Solid | FeCO3 | −750.6 |
| Iron(III) chloride | Solid | FeCl3 | −399.4 |
| Iron(II) oxide (Wüstite) | Solid | FeO | −272 |
| Iron(II,III) oxide (Magnetite) | Solid | Fe3O4 | −1118.4 |
| Iron(III) oxide (Hematite) | Solid | Fe2O3 | −824.2 |
| Iron(II) sulfate | Solid | FeSO4 | −929 |
| Iron(III) sulfate | Solid | Fe2(SO4)3 | −2583 |
| Iron(II) sulfide | Solid | FeS | −102 |
| Pyrite | Solid | FeS2 | −178 |
| Lead | Solid | Pb | 0 |
| Lead dioxide | Solid | PbO2 | −277 |
| Lead sulfide | Solid | PbS | −100 |
| Lead sulfate | Solid | PbSO4 | −920 |
| Lead(II) nitrate | Solid | Pb(NO3)2 | −452 |
| Lead(II) sulfate | Solid | PbSO4 | −920 |
| Lithium fluoride | Solid | LiF | −616.93 |
| Magnesium | Solid | Mg | 0 |
| Magnesium ion | Aqueous | Mg | −466.85 |
| Magnesium carbonate | Solid | MgCO3 | −1095.797 |
| Magnesium chloride | Solid | MgCl2 | −641.8 |
| Magnesium hydroxide | Solid | Mg(OH)2 | −924.54 |
| Magnesium hydroxide | Aqueous | Mg(OH)2 | −926.8 |
| Magnesium oxide | Solid | MgO | −601.6 |
| Magnesium sulfate | Solid | MgSO4 | −1278.2 |
| Manganese | Solid | Mn | 0 |
| Manganese(II) oxide | Solid | MnO | −384.9 |
| Manganese(IV) oxide | Solid | MnO2 | −519.7 |
| Manganese(III) oxide | Solid | Mn2O3 | −971 |
| Manganese(II,III) oxide | Solid | Mn3O4 | −1387 |
| Permanganate | Aqueous | MnO 4 |
−543 |
| Mercury(II) oxide (red) | Solid | HgO | −90.83 |
| Mercury sulfide (red, cinnabar) | Solid | HgS | −58.2 |
| Nitrogen | Gas | N2 | 0 |
| Ammonia (ammonium hydroxide) | Aqueous | NH3 (NH4OH) | −80.8 |
| Ammonia | Gas | NH3 | −46.1 |
| Ammonium nitrate | Solid | NH4NO3 | −365.6 |
| Ammonium chloride | Solid | NH4Cl | −314.55 |
| Nitrogen dioxide | Gas | NO2 | 33.2 |
| Hydrazine | Gas | N2H4 | 95.4 |
| Hydrazine | Liquid | N2H4 | 50.6 |
| Nitrous oxide | Gas | N2O | 82.05 |
| Nitric oxide | Gas | NO | 90.29 |
| Dinitrogen tetroxide | Gas | N2O4 | 9.16 |
| Dinitrogen pentoxide | Solid | N2O5 | −43.1 |
| Dinitrogen pentoxide | Gas | N2O5 | 11.3 |
| Nitric acid | Aqueous | HNO3 | −207 |
| Monatomic oxygen | Gas | O | 249 |
| Oxygen | Gas | O2 | 0 |
| Ozone | Gas | O3 | 143 |
| White phosphorus | Solid | P4 | 0 |
| Red phosphorus | Solid | P | −17.4 |
| Black phosphorus | Solid | P | −39.3 |
| Phosphorus trichloride | Liquid | PCl3 | −319.7 |
| Phosphorus trichloride | Gas | PCl3 | −278 |
| Phosphorus pentachloride | Solid | PCl5 | −440 |
| Phosphorus pentachloride | Gas | PCl5 | −321 |
| Phosphorus pentoxide | Solid | P2O5 | −1505.5 |
| Potassium bromide | Solid | KBr | −392.2 |
| Potassium carbonate | Solid | K2CO3 | −1150 |
| Potassium chlorate | Solid | KClO3 | −391.4 |
| Potassium chloride | Solid | KCl | −436.68 |
| Potassium fluoride | Solid | KF | −562.6 |
| Potassium oxide | Solid | K2O | −363 |
| Potassium nitrate | Solid | KNO3 | −494.5 |
| Potassium perchlorate | Solid | KClO4 | −430.12 |
| Silicon | Gas | Si | 368.2 |
| Silicon carbide | Solid | SiC | −74.4, −71.5 |
| Silicon tetrachloride | Liquid | SiCl4 | −640.1 |
| Silica (Quartz) | Solid | SiO2 | −910.86 |
| Silver bromide | Solid | AgBr | −99.5 |
| Silver chloride | Solid | AgCl | −127.01 |
| Silver iodide | Solid | AgI | −62.4 |
| Silver oxide | Solid | Ag2O | −31.1 |
| Silver sulfide | Solid | Ag2S | −31.8 |
| Sodium | Solid | Na | 0 |
| Sodium | Gas | Na | 107.5 |
| Sodium bicarbonate | Solid | NaHCO3 | −950.8 |
| Sodium carbonate | Solid | Na2CO3 | −1130.77 |
| Sodium chloride | Aqueous | NaCl | −407.27 |
| Sodium chloride | Solid | NaCl | −411.12 |
| Sodium chloride | Liquid | NaCl | −385.92 |
| Sodium chloride | Gas | NaCl | −181.42 |
| Sodium chlorate | Solid | NaClO3 | −365.4 |
| Sodium fluoride | Solid | NaF | −569.0 |
| Sodium hydroxide | Aqueous | NaOH | −469.15 |
| Sodium hydroxide | Solid | NaOH | −425.93 |
| Sodium hypochlorite | Solid | NaOCl | −347.1 |
| Sodium nitrate | Aqueous | NaNO3 | −446.2 |
| Sodium nitrate | Solid | NaNO3 | −424.8 |
| Sodium oxide | Solid | Na2O | −414.2 |
| Sulfur (monoclinic) | Solid | S8 | 0.3 |
| Sulfur (rhombic) | Solid | S8 | 0 |
| Hydrogen sulfide | Gas | H2S | −20.63 |
| Sulfur dioxide | Gas | SO2 | −296.84 |
| Sulfur trioxide | Gas | SO3 | −395.7 |
| Sulfuric acid | Liquid | H2SO4 | −814 |
| Titanium | Gas | Ti | 468 |
| Titanium tetrachloride | Gas | TiCl4 | −763.2 |
| Titanium tetrachloride | Liquid | TiCl4 | −804.2 |
| Titanium dioxide | Solid | TiO2 | −944.7 |
| Zinc | Gas | Zn | 130.7 |
| Zinc chloride | Solid | ZnCl2 | −415.1 |
| Zinc oxide | Solid | ZnO | −348.0 |
| Zinc sulfate | Solid | ZnSO4 | −980.14 |
Aliphatic hydrocarbons
| Formula | Name | ΔfH /(kcal/mol) | ΔfH /(kJ/mol) |
|---|---|---|---|
| Straight-chain | |||
| CH4 | Methane | −17.9 | −74.9 |
| C2H6 | Ethane | −20.0 | −83.7 |
| C2H4 | Ethylene | 12.5 | 52.5 |
| C2H2 | Acetylene | 54.2 | 226.8 |
| C3H8 | Propane | −25.0 | −104.6 |
| C4H10 | n-Butane | −30.0 | −125.5 |
| C5H12 | n-Pentane | −35.1 | −146.9 |
| C6H14 | n-Hexane | −40.0 | −167.4 |
| C7H16 | n-Heptane | −44.9 | −187.9 |
| C8H18 | n-Octane | −49.8 | −208.4 |
| C9H20 | n-Nonane | −54.8 | −229.3 |
| C10H22 | n-Decane | −59.6 | −249.4 |
| C4 Alkane branched isomers | |||
| C4H10 | Isobutane (methylpropane) | −32.1 | −134.3 |
| C5 Alkane branched isomers | |||
| C5H12 | Neopentane (dimethylpropane) | −40.1 | −167.8 |
| C5H12 | Isopentane (methylbutane) | −36.9 | −154.4 |
| C6 Alkane branched isomers | |||
| C6H14 | 2,2-Dimethylbutane | −44.5 | −186.2 |
| C6H14 | 2,3-Dimethylbutane | −42.5 | −177.8 |
| C6H14 | 2-Methylpentane (isohexane) | −41.8 | −174.9 |
| C6H14 | 3-Methylpentane | −41.1 | −172.0 |
| C7 Alkane branched isomers | |||
| C7H16 | 2,2-Dimethylpentane | −49.2 | −205.9 |
| C7H16 | 2,2,3-Trimethylbutane | −49.0 | −205.0 |
| C7H16 | 3,3-Dimethylpentane | −48.1 | −201.3 |
| C7H16 | 2,3-Dimethylpentane | −47.3 | −197.9 |
| C7H16 | 2,4-Dimethylpentane | −48.2 | −201.7 |
| C7H16 | 2-Methylhexane | −46.5 | −194.6 |
| C7H16 | 3-Methylhexane | −45.7 | −191.2 |
| C7H16 | 3-Ethylpentane | −45.3 | −189.5 |
| C8 Alkane branched isomers | |||
| C8H18 | 2,3-Dimethylhexane | −55.1 | −230.5 |
| C8H18 | 2,2,3,3-Tetramethylbutane | −53.9 | −225.5 |
| C8H18 | 2,2-Dimethylhexane | −53.7 | −224.7 |
| C8H18 | 2,2,4-Trimethylpentane (isooctane) | −53.5 | −223.8 |
| C8H18 | 2,5-Dimethylhexane | −53.2 | −222.6 |
| C8H18 | 2,2,3-Trimethylpentane | −52.6 | −220.1 |
| C8H18 | 3,3-Dimethylhexane | −52.6 | −220.1 |
| C8H18 | 2,4-Dimethylhexane | −52.4 | −219.2 |
| C8H18 | 2,3,4-Trimethylpentane | −51.9 | −217.1 |
| C8H18 | 2,3,3-Trimethylpentane | −51.7 | −216.3 |
| C8H18 | 2-Methylheptane | −51.5 | −215.5 |
| C8H18 | 3-Ethyl-3-Methylpentane | −51.4 | −215.1 |
| C8H18 | 3,4-Dimethylhexane | −50.9 | −213.0 |
| C8H18 | 3-Ethyl-2-Methylpentane | −50.4 | −210.9 |
| C8H18 | 3-Methylheptane | −60.3 | −252.5 |
| C8H18 | 4-Methylheptane | ? | ? |
| C8H18 | 3-Ethylhexane | ? | ? |
| C9 Alkane branched isomers (selected) | |||
| C9H20 | 2,2,4,4-Tetramethylpentane | −57.8 | −241.8 |
| C9H20 | 2,2,3,3-Tetramethylpentane | −56.7 | −237.2 |
| C9H20 | 2,2,3,4-Tetramethylpentane | −56.6 | −236.8 |
| C9H20 | 2,3,3,4-Tetramethylpentane | −56.4 | −236.0 |
| C9H20 | 3,3-Diethylpentane | −55.7 | −233.0 |
Other organic compounds
| Species | Phase | Chemical formula | ΔfH /(kJ/mol) |
|---|---|---|---|
| Acetone | Liquid | C3H6O | −248.4 |
| Benzene | Liquid | C6H6 | 48.95 |
| Benzoic acid | Solid | C7H6O2 | −385.2 |
| Carbon tetrachloride | Liquid | CCl4 | −135.4 |
| Carbon tetrachloride | Gas | CCl4 | −95.98 |
| Ethanol | Liquid | C2H5OH | −277.0 |
| Ethanol | Gas | C2H5OH | −235.3 |
| Glucose | Solid | C6H12O6 | −1271 |
| Isopropanol | Gas | C3H7OH | −318.1 |
| Methanol (methyl alcohol) | Liquid | CH3OH | −238.4 |
| Methanol (methyl alcohol) | Gas | CH3OH | −201.0 |
| Methyl linoleate (Biodiesel) | Gas | C19H34O2 | −356.3 |
| Sucrose | Solid | C12H22O11 | −2226.1 |
| Trichloromethane (Chloroform) | Liquid | CHCl3 | −134.47 |
| Trichloromethane (Chloroform) | Gas | CHCl3 | −103.18 |
| Vinyl chloride | Solid | C2H3Cl | −94.12 |
See also
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "standard pressure". doi:10.1351/goldbook.S05921
- Oxtoby, David W; Pat Gillis, H; Campion, Alan (2011). Principles of Modern Chemistry. Cengage Learning. p. 547. ISBN 978-0-8400-4931-5.
- Moore, Stanitski, and Jurs. Chemistry: The Molecular Science. 3rd edition. 2008. ISBN 0-495-10521-X. pages 320–321.
- "Enthalpies of Reaction". www.science.uwaterloo.ca. Archived from the original on 25 October 2017. Retrieved 2 May 2018.
- ^ Housecroft, C. E.; Sharpe, A. G. (2004). Inorganic Chemistry (2nd ed.). Prentice Hall. p. 392. ISBN 978-0-13-039913-7.
- Green, D.W., ed. (2007). Perry's Chemical Engineers' Handbook (8th ed.). Mcgraw-Hill. pp. 2–191. ISBN 9780071422949.
- Kleykamp, H. (1998). "Gibbs Energy of Formation of SiC: A contribution to the Thermodynamic Stability of the Modifications". Berichte der Bunsengesellschaft für physikalische Chemie. 102 (9): 1231–1234. doi:10.1002/bbpc.19981020928.
- "Silicon Carbide, Alpha (SiC)". March 1967. Retrieved 5 February 2019.
- Zumdahl, Steven (2009). Chemical Principles (6th ed.). Boston. New York: Houghton Mifflin. pp. 384–387. ISBN 978-0-547-19626-8.










 is determined to be −74.8 kJ/mol. The negative sign shows that the reaction, if it were to proceed, would be
is determined to be −74.8 kJ/mol. The negative sign shows that the reaction, if it were to proceed, would be 
 :
:

 is an element in its standard state, so that
is an element in its standard state, so that  , and the heat of reaction is simplified to
, and the heat of reaction is simplified to

 .
.