| Haloform reaction | |
|---|---|
| Named after | Adolf Lieben |
| Reaction type | Substitution reaction |
| Identifiers | |
| Organic Chemistry Portal | haloform-reaction |
| RSC ontology ID | RXNO:0000689 |
In chemistry, the haloform reaction (also referred to as the Lieben haloform reaction) is a chemical reaction in which a haloform (CHX3, where X is a halogen) is produced by the exhaustive halogenation of an acetyl group (R−C(=O)CH3, where R can be either a hydrogen atom, an alkyl or an aryl group), in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups (R−C(=O)OH) or to produce chloroform (CHCl3), bromoform (CHBr3), or iodoform (CHI3). Note that fluoroform (CHF3) can't be prepared in this way.

Mechanism
In the first step, the halogen dis-proportionates in the presence of hydroxide to give the halide and hypohalite.
If a secondary alcohol is present, it is oxidized to a ketone by the hypohalite:

If a methyl ketone is present, it reacts with the hypohalite in a three-step process:
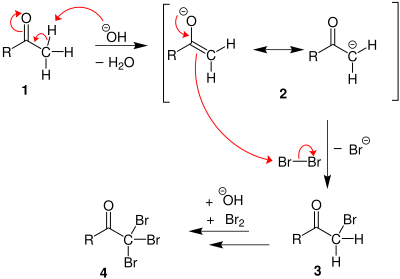
1. Under basic conditions, the ketone undergoes keto-enol tautomerisation. The enolate undergoes electrophilic attack by the hypohalite (containing a halogen with a formal +1 charge).
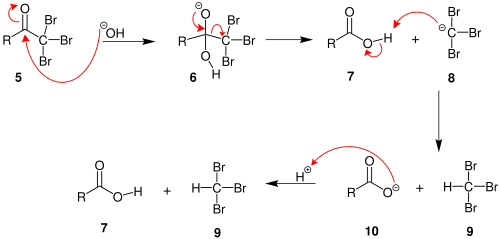
2. When the α(alpha) position has been exhaustively halogenated, the molecule reacts with hydroxide, with CX3 being the leaving group stabilized by three electron-withdrawing groups. In the third step the CX3 anion abstracts a proton from either the solvent or the carboxylic acid formed in the previous step, and forms the haloform. At least in some cases (chloral hydrate) the reaction may stop and the intermediate product isolated if conditions are acidic and hypohalite is used.
Scope
Substrates are broadly limited to methyl ketones and secondary alcohols oxidizable to methyl ketones, such as isopropanol. The only primary alcohol and aldehyde to undergo this reaction are ethanol and acetaldehyde, respectively. 1,3-Diketones such as acetylacetone also undergo this reaction. β-ketoacids such as acetoacetic acid will also give the test upon heating. Acetyl chloride and acetamide do not undergo this reaction. The halogen used may be chlorine, bromine, iodine or sodium hypochlorite. Fluoroform (CHF3) cannot be prepared by this method as it would require the presence of the highly unstable hypofluorite ion. However ketones with the structure RCOCF3 do cleave upon treatment with base to produce fluoroform; this is equivalent to the second and third steps in the process shown above.
Applications
Laboratory scale

This reaction forms the basis of the iodoform test which was commonly used in history as a chemical test to determine the presence of a methyl ketone, or a secondary alcohol oxidizable to a methyl ketone. When iodine and sodium hydroxide are used as the reagents a positive reaction gives iodoform, which is a solid at room temperature and tends to precipitate out of solution causing a distinctive cloudiness.
In organic chemistry, this reaction may be used to convert a terminal methyl ketone into the analogous carboxylic acid.
Industrially
It was formerly used to produce iodoform, bromoform, and even chloroform industrially.
A variant of this reaction is used to manufacture deuterated chloroform, in reaction of hexachloroacetone with heavy water catalysed by base:
- O=C(CCl3)2 + D2O → 2 CDCl3 + CO2
Further variant uses decomposition of calcium trichloroacetate in heavy water:
- Ca(CCl3CO2)2 + D2O → 2 CDCl3 + CaCO3 + CO2
As a by-product of water chlorination
Water chlorination can result in the formation of haloforms if the water contains suitable reactive impurities (e.g. humic acid). There is a concern that such reactions may lead to the presence of carcinogenic compounds in drinking water.
History
The haloform reaction is one of the oldest organic reactions known. In 1822, Georges-Simon Serullas added potassium metal to a solution of iodine in ethanol and water to form potassium formate and iodoform, called in the language of that time hydroiodide of carbon. In 1832, Justus von Liebig reported the reaction of chloral with calcium hydroxide to form chloroform and calcium formate. The reaction was rediscovered by Adolf Lieben in 1870. The iodoform test is also called the Lieben iodoform reaction. A review of the haloform reaction with a history section was published in 1934.
References
- March, Jerry; Smith, Michael B. (2007). Knipe, A.C. (ed.). March's Advanced Organic Chemistry Reactions, Mechanisms, and Structure (6th ed.). Hoboken: John Wiley & Sons. p. 484. ISBN 9780470084946.
- ^ Reynold C. Fuson and Benton A. Bull (1934). "The Haloform Reaction". Chemical Reviews. 15 (3): 275–309. doi:10.1021/cr60052a001.
- Chakrabartty, in Trahanovsky, Oxidation in Organic Chemistry, pp. 343–370, Academic Press, New York, 1978
- Bain, Ryan M.; Pulliam, Christopher J.; Raab, Shannon A.; Cooks, R. Graham (2016). "Chemical Synthesis Accelerated by Paper Spray: The Haloform Reaction". Journal of Chemical Education. 93 (2): 340–344. Bibcode:2016JChEd..93..340B. doi:10.1021/acs.jchemed.5b00263. ISSN 0021-9584.
- Paulsen, P. J.; Cooke, W. D. (1 September 1963). "Preparation of Deuterated Solvents for Nuclear Magnetic Resonance Spectrometry". Analytical Chemistry. 35 (10): 1560. doi:10.1021/ac60203a072.
- Zaharani, Lia; Johan, Mohd Rafie Bin; Khaligh, Nader Ghaffari (2022). "Cost and Energy Saving Process for the Laboratory-Scale Production of Chloroform-d". Organic Process Research & Development. 26 (11): 3126–3129. doi:10.1021/acs.oprd.2c00260. S2CID 253071632.
- Earing, Mason H.; Cloke, John B. (1951). "A New Synthesis of Chloroform-d1". Journal of the American Chemical Society. 73 (2): 769–770. doi:10.1021/ja01146a080.
- Rook, Johannes J. (1977). "Chlorination reactions of fulvic acids in natural waters". Environmental Science & Technology. 11 (5): 478–482. Bibcode:1977EnST...11..478R. doi:10.1021/es60128a014. ISSN 0013-936X.
- Reckhow, David A.; Singer, Philip C.; Malcolm, Ronald L. (1990). "Chlorination of humic materials: byproduct formation and chemical interpretations". Environmental Science & Technology. 24 (11): 1655–1664. Bibcode:1990EnST...24.1655R. doi:10.1021/es00081a005. ISSN 0013-936X.
- Boorman, GA (February 1999). "Drinking water disinfection byproducts: review and approach to toxicity evaluation". Environmental Health Perspectives. 107 (Suppl 1): 207–17. doi:10.1289/ehp.99107s1207. PMC 1566350. PMID 10229719.
- László Kürti and Barbara Czakó (2005). Strategic Applications of Named Reactions in Organic Synthesis. Amsterdam: Elsevier. ISBN 0-12-429785-4.
- Surellas, Georges-Simon (May 1822). Notes sur l'Hydriodate de potasse et l'Acide hydriodique. – Hydriodure de carbone; moyen d'obtenir, à l'instant, ce composé triple [Notes on the hydroiodide of potassium and on hydroiodic acid – hydroiodide of carbon; means of obtaining instantly this compound of three elements] (in French). Metz, France: Antoine. On pages 17–20, Surellas produced iodoform by passing a mixture of iodine vapor and steam over red-hot coals. However, later, on pages 28–29, he produced iodoform by adding potassium metal to a solution of iodine in ethanol (which also contained some water).
- Liebig, Justus von (1832). "Ueber die Verbindungen, welche durch die Einwirkung des Chlors auf Alkohol, Aether, ölbildendes Gas und Essiggeist entstehen" [On the compounds which arise by the reaction of chlorine with base , ether , oil-forming gas , and spirit of vinegar ]. Annalen der Physik und Chemie. 2nd series. 100 (2): 243–295. Bibcode:1832AnP...100..243L. doi:10.1002/andp.18321000206.
On pages 259–265, Liebig describes Chlorkohlenstoff ("carbon chloride", chloroform), but on p. 264, Liebig incorrectly states that the empirical formula of chloroform is C2Cl5. From p. 259: "Chlorkohlenstoff. Man erhält diese neue Verbindung, wenn man Chloral mit ätzenden Alkalien, Kalkmilch oder Barytwasser in Ueberschuss vermischt und das Gemenge destillirt." (Chloroform. One obtains this new compound when one mixes chloral with an excess of caustic alkalies, milk of lime or barite water , and distills the mixture.) - See:
- Lieben, Adolf (1870). "Ueber Entstehung von Jodoform und Anwendung dieser Reaction in der chemischen Analyse" [On the formation of iodoform and the application of this reaction to chemical analysis]. Annalen der Chemie. Supplementband. (in German). 7: 218–236.
- Lieben, Adolf (1870). "Nachschrift zur Abhandlung über Entstehung von Jodoform und Anwendung dieser Reaction in der chemischen Analyse" [Postscript to the article on the formation of iodoform and the application of this reaction to chemical analysis]. Annalen der Chemie. Supplementband. (in German). 7: 377–378.