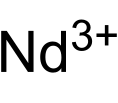 
| |
| Names | |
|---|---|
| IUPAC names Dineodymium trisulfide | |
| Other names Neodymium sulfide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.031.642 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | Nd2S3 |
| Molar mass | 384.66 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Neodymium(III) sulfide is a inorganic chemical compound with the formula Nd2S3 composed of a two neodymium atoms in the +3 oxidation state and three sulfur atoms in the -2 oxidation state. Like other rare earth sulfides, neodymium(III) sulfide is used as a high-performance inorganic pigment.
Preparation
Neodymium(III) sulfide can directly be produced by reacting neodymium with sulfur:
- 2Nd + 3S → Nd2S3
It can also be produced by sulfidizing neodymium oxide with H2S at 1450 °C:
- Nd2O3 + 3 H2S → Nd2S3 + 3 H2O
Properties
Neodymium(III) sulfide is (as γ-form) a light green solid. The compound comes in three forms. The α-form has an orthorhombic crystal structure, the β form has a tetragonal crystal structure, and the γ form has a cubic crystal structure. At 1650 °C in a vacuum, the γ compound decomposes to form neodymium monosulfide.
Neodymium(III) sulfide has a high melting point and a lot of polymorphic forms which make it difficult to grow. When heated, neodymium sulfide can lose sulfur atoms and can form a range of compositions between Nd2S3 and Nd3S4. Neodymium(III) sulfide is an electrical insulator.
See also
References
- "Neodymium sulfide (Nd2S3)". pubchem.ncbi.nlm.nih.gov. Retrieved 11 April 2022.
- ^ Uspenskaya, S. I.; Eliseev, A. A.; Fedorov, A. A. (1975), Sheftal’, N. N.; Givargizov, E. I. (eds.), "Vapor Growth of Lanthanum and Neodymium Sulfide Crystals", РОСТ КРИСТАЛЛОВ/Rost Kristallov/Growth of Crystals, Boston, MA: Springer New York, pp. 257–260, doi:10.1007/978-1-4684-1689-3_55, ISBN 978-1-4684-1691-6, retrieved 2023-04-22
- ^ Faulkner, Edwin B.; Schwartz, Russell J. (2009-03-09). High Performance Pigments. John Wiley & Sons. ISBN 978-3-527-62692-2.
- ^ Cotton, Simon (2006). Lanthanide and Actinide Chemistry. John Wiley & Sons Ltd.
- A. W. Sleight and D. P. Kelly (1973), Aaron Wold and John K. Ruff (ed.), Rare-earth sesquisulfides, Ln2S3, Inorganic Syntheses (in German), vol. 14, McGraw-Hill Book Company, Inc., pp. 152–155
- ^ Meyer, G.; Morss, Lester R. (1990-12-31). Synthesis of Lanthanide and Actinide Compounds. Springer Science & Business Media. ISBN 978-0-7923-1018-1.
| Neodymium compounds | |
|---|---|
| Nd(II) | |
| Nd(III) | |
| Nd(IV) | |
| Sulfides (S) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||